Abstract
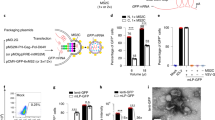
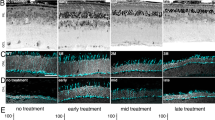
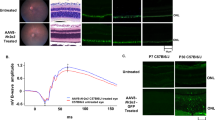
X-linked retinoschisis, a disease characterized by splitting of the retina, is caused by mutations in the retinoschisin gene, which encodes a putative secreted cell adhesion protein. Currently, there is no effective treatment for retinoschisis, though viral vector-mediated gene replacement therapies offer promise. We used intravitreal delivery of three different AAV vectors to target delivery of the RS1 gene to Müller glia, photoreceptors or multiple cell types throughout the retina. Müller glia radially span the entire retina, are accessible from the vitreous, and remain intact throughout progression of the disease. However, photoreceptors, not glia, normally secrete retinoschisin. We compared the efficacy of rescue mediated by retinoschisin secretion from these specific subtypes of retinal cells in the Rs1h−/− mouse model of retinoschisis. Our results indicate that all three vectors deliver the RS1 gene, and that several cell types can secrete retinoschisin, leading to transport of the protein across the retina. The greatest long-term rescue was observed when photoreceptors produce retinoschisin. Similar rescue was observed with photoreceptor-specific or generalized expression, although photoreceptor secretion may contribute to rescue in the latter case. These results collectively point to the importance of cell targeting and appropriate vector choice in the success of retinal gene therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sauer CG, Gehrig A, Warneke-Wittstock R, Marquardt A, Ewing CC, Gibson A et al. Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nat Genet 1997; 17: 164–170.
George ND, Yates JR, Moore AT . X linked retinoschisis. Br J Opthalmol 1995; 79: 697–702.
Sikkink SK, Biswas S, Parry NRA, Stanga PE, Trump D . X-linked retinoschisis: an update. J Med Genet 2007; 44: 225–232.
Forsius HH, Krause UU, Helve JJ, Vuopala VV, Mustonen EE, Vainio-Mattila BB et al. Visual acuity in 183 cases of X-chromosomal retinoschisis. Can J Ophthalmol 1973; 8: 385–393.
Vijayasarathy C, Takada Y, Zeng Y, Bush RA, Sieving PA . Retinoschisin is a peripheral membrane protein with affinity for anionic phospholipids and affected by divalent cations. Invest Ophthalmol Vis Sci 2007; 48: 991–1000.
Molday LL, Wu WWH, Molday RS . Retinoschisin (RS1), the protein encoded by the X-linked retinoschisis gene, is anchored to the surface of retinal photoreceptor and bipolar cells through its interactions with a Na/K ATPase-SARM1 complex. J Biol Chem 2007; 282: 32792–32801.
Weber BHF, Schrewe H, Molday LL, Gehrig A, White KL, Seeliger MW et al. Inactivation of the murine X-linked juvenile retinoschisis gene, Rs1h, suggests a role of retinoschisin in retinal cell layer organization and synaptic structure. Proc Natl Acad Sci USA 2002; 99: 6222–6227.
Gehrig A, Janssen A, Horling F, Grimm C, Weber BHF . The role of caspases in photoreceptor cell death of the retinoschisin-deficient mouse. Cytogenet Genome Res 2006; 115: 35–44.
Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med 2013; 5: 189ra76.
Park TK, Wu Z, Kjellstrom S, Zeng Y, Bush RA, Sieving PA et al. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Therapy 2009; 16: 916–926.
Takada Y, Vijayasarathy C, Zeng Y, Kjellstrom S, Bush RA, Sieving PA . Synaptic pathology in retinoschisis knockout (Rs1-/y) mouse retina and modification by rAAV-Rs1 gene delivery. Invest Ophthalmol Vis Sci 2008; 49: 3677–3686.
Janssen A, Min SH, Molday LL, Tanimoto N, Seeliger MW, Hauswirth WW et al. Effect of late-stage therapy on disease progression in AAV-mediated rescue of photoreceptor cells in the retinoschisin-deficient mouse. Mol Ther 2008; 16: 1010–1017.
Kjellstrom S, Bush RA, Zeng Y, Takada Y, Sieving PA . Retinoschisin gene therapy and natural history in the Rs1h-KO mouse: long-term rescue from retinal degeneration. Invest Ophthalmol Vis Sci 2007; 48: 3837–3845.
Molday LL, Min S-H, Seeliger MW, Wu WWH, Dinculescu A, Timmers AM et al. Disease mechanisms and gene therapy in a mouse model for X-linked retinoschisis. Adv Exp Med Biol 2006; 572: 283–289.
Min SH, Molday LL, Seeliger MW, Dinculescu A, Timmers AM, Janssen A et al. Prolonged recovery of retinal structure/function after gene therapy in an Rs1h-deficient mouse model of x-linked juvenile retinoschisis. Mol Ther 2005; 12: 644–651.
Zeng Y, Takada Y, Kjellstrom S, Hiriyanna K, Tanikawa A, Wawrousek E et al. RS-1 Gene delivery to an adult Rs1h knockout mouse model restores ERG b-wave with Reversal of the electronegative waveform of X-linked retinoschisis. Invest Ophthalmol Vis Sci 2004; 45: 3279–3285.
Klimczak RR, Koerber JT, Dalkara D, Flannery JG, Schaffer DV . A novel adeno-associated viral variant for efficient and selective intravitreal transduction of rat Müller cells. PLoS One 2009; 4: e7467.
Koerber JT, Klimczak R, Jang J-H, Dalkara D, Flannery JG, Schaffer DV . Molecular evolution of adeno-associated virus for enhanced glial gene delivery. Mol Ther 2009; 17: 2088–2095.
Reid SNM, Yamashita C, Farber DB . Retinoschisin, a photoreceptor-secreted protein, and its interaction with bipolar and muller cells. J Neurosci 2003; 23: 6030–6040.
Reid SNM, Farber DB . Glial transcytosis of a photoreceptor-secreted signaling protein, retinoschisin. Glia 2005; 49: 397–406.
Friedrich U, Stöhr H, Hilfinger D, Loenhardt T, Schachner M, Langmann T et al. The Na/K-ATPase is obligatory for membrane anchorage of retinoschisin, the protein involved in the pathogenesis of X-linked juvenile retinoschisis. Hum Mol Genet 2011; 20: 1132–1142.
Wetzel RK, Arystarkhova E, Sweadner KJ . Cellular and subcellular specification of Na,K-ATPase alpha and beta isoforms in the postnatal development of mouse retina. J Neurosci 1999; 19: 9878–9889.
Takada Y, Fariss RN, Tanikawa A, Zeng Y, Carper D, Bush R et al. A retinal neuronal developmental wave of retinoschisin expression begins in ganglion cells during layer formation. Invest Ophthalmol Vis Sci 2004; 45: 3302–3312.
Stieger K, Colle M-A, Dubreil L, Mendes-Madeira A, Weber M, Le Meur G et al. Subretinal delivery of recombinant AAV serotype 8 vector in dogs results in gene transfer to neurons in the brain. Mol Ther 2008; 16: 916–923.
Grieger JC, Choi VW, Samulski RJ . Production and characterization of adeno-associated viral vectors. Nat Protoc 2006; 1: 1412–1428.
Aurnhammer C, Haase M, Muether N, Hausl M, Rauschhuber C, Huber I et al. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum Gene Ther Methods 2012; 23: 18–28.
Acknowledgements
We thank Robert Molday for providing the 3R10 anti-RS1 antibody. We thank Bernhardt Weber and Bill Hauswirth for supplying the mouse model of XLRS. We thank Günter Niemeyer, Greg Nielsen and Matt LaVail for valuable advice on ERG recordings. We also thank Tim Day for assisting with plasmid cloning, and Jonathan Jui for helping with immunohistochemistry. This work was supported by funding from the NIH and FFB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
DD, JGF and DVS are patent holders on ShH10 for gene delivery to the retina. LCB, DD, MV, JGF and DVS are patent holders on 7m8 for delivery of gene products to retinal cells.
Rights and permissions
About this article
Cite this article
Byrne, L., Öztürk, B., Lee, T. et al. Retinoschisin gene therapy in photoreceptors, Müller glia or all retinal cells in the Rs1h−/− mouse. Gene Ther 21, 585–592 (2014). https://doi.org/10.1038/gt.2014.31
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2014.31