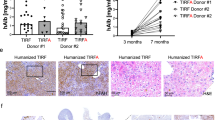
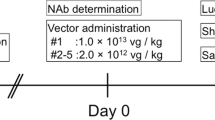
Abstract
Adeno-associated virus (AAV) vectors are highly efficient for liver-targeted gene delivery in murine models and show promise in early phase human clinical trials. This efficiency is capsid-dependent and was only achieved after discovery that the AAV2 vector genome could be trans-encapsidated into the capsids of other AAV serotypes. This confers novel host-vector biology and target tissue tropism. Optimal exploitation of the growing number of AAV vector pseudo-serotypes, however, requires detailed context-dependent characterisation of transduction performance. In this study, we compared the pattern and efficiency of gene delivery to the adult mouse liver following intraportal and intraperitoneal injection of vectors pseudo-serotyped with known hepatotropic capsids from AAV type 7, 8, 9 and rhesus 10. Vectors pseudo-serotyped with these hepatotropic capsids proved relatively efficient irrespective of administration route, with higher transgene expression in males despite equivalent vector genome delivery in females. Transgene expression was predominantly centrilobular in contrast to the AAV2 capsid, which gave a periportal pattern of expression. Most intriguingly, vector genome performance appeared to be delivery route-dependent, consistent with the possibility of in vivo capsid modification. These data not only inform the experimental use of AAV vectors, but also provide insight into novel aspects of host-vector biology requiring further focused analysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Alexander IE, Cunningham SC, Logan GJ, Christodoulou J . Potential of AAV vectors in the treatment of metabolic disease. Gene Therapy 2008; 15: 831–839.
Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood 2006; 108: 3321–3328.
Nathwani AC, Rosales C, McIntosh J, Rastegarlari G, Nathwani D, Raj D et al. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol Ther 2011; 19: 876–885.
Wang L, Bell P, Lin J, Calcedo R, Tarantal AF, Wilson JM . AAV8-mediated hepatic gene transfer in infant rhesus monkeys (Macaca mulatta). Mol Ther 2011; 19: 2012–2020.
Jiang H, Lillicrap D, Patarroyo-White S, Liu T, Qian X, Scallan CD et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood 2006; 108: 107–115.
Wang L, Calcedo R, Nichols TC, Bellinger DA, Dillow A, Verma IM et al. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood 2005; 105: 3079–3086.
Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011; 365: 2357–2365.
Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, Muruve DA . Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol 2002; 76: 4580–4590.
Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol 2002; 76: 791–801.
Thomas CE, Storm TA, Huang Z, Kay MA . Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol 2004; 78: 3110–3122.
Di Pasquale G, Chiorini JA . AAV transcytosis through barrier epithelia and endothelium. Mol Ther 2006; 13: 506–516.
Mitchell AM, Nicolson SC, Warischalk JK, Samulski RJ . AAV’s anatomy: roadmap for optimizing vectors for translational success. Curr Gene Ther 2010; 10: 319–340.
Manno CS, Arruda VR, Pierce GF, Glader B, Ragni M, Rasko J et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nature Med 2006; 12: 342–347.
Mingozzi F, High KA . Immune responses to AAV in clinical trials. Curr Gene Ther 2007; 7: 316–324.
Sack BK, Herzog RW . Evading the immune response upon in vivo gene therapy with viral vectors. Curr Opin Mol Ther 2009; 11: 493–503.
Cunningham SC, Dane AP, Spinoulas A, Alexander IE . Gene delivery to the juvenile mouse liver using AAV2/8 Vectors. Mol Ther 2008; 16: 1081–1088.
Moscioni D, Morizono H, McCarter RJ, Stern A, Cabrera-Luque J, Hoang A et al. Long-term correction of ammonia metabolism and prolonged survival in ornithine transcarbamylase-deficient mice following liver-directed treatment with novel adeno-associated viral vectors. Mol Ther 2006; 14: 25–33.
Dane AP, Cunningham SC, Graf NS, Alexander IE . Sexually dimorphic patterns of episomal rAAV genome persistence in the adult mouse liver and correlation with hepatocellular proliferation. Mol Ther 2009; 17: 1548–1554.
Bell P, Wang L, Gao G, Haskins ME, Tarantal AF, McCarter RJ et al. Inverse zonation of hepatocyte transduction with AAV vectors between mice and non-human primates. Mol Genet Metab 2011; 104: 395–403.
Jungermann K . Zonation of metabolism and gene expression in liver. Histochem Cell Biol 1995; 103: 81–91.
Davidoff AM, Ng CY, Zhou J, Spence Y, Nathwani AC . Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood 2003; 102: 480–488.
De BP, Heguy A, Hackett NR, Ferris B, Leopold PL, Lee J et al. High levels of persistent expression of alpha1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol Ther 2006; 13: 67–76.
Sun B, Zhang H, Franco LM, Brown T, Bird A, Schneider A et al. Correction of glycogen storage disease type II by an adeno-associated virus vector containing a muscle-specific promoter. Mol Ther 2005; 11: 889–898.
Lonning SM, Ford CA, Taylor KM, Hempel DM, Plog M, Ziegler RJ et al. Influence of gender-specific hormones on adeno-associated virus vector gene transfer and expression. Mol Ther 2002; 5: S2–S2.
Sun B, Zhang H, Franco LM, Young SP, Schneider A, Bird A et al. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol Ther 2005; 11: 57–65.
Halbert DN, Cutt JR, Shenk T . Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol 1985; 56: 250–257.
Ding W, Zhang L, Yan Z, Engelhardt JF . Intracellular trafficking of adeno-associated viral vectors. Gene Therapy 2005; 12: 873–880.
Wang J, Xie J, Lu H, Chen L, Hauck B, Samulski RJ et al. Existence of transient functional double-stranded DNA intermediates during recombinant AAV transduction. Proc Natl Acad Sci USA 2007; 104: 13104–13109.
Keiser NW, Yan Z, Zhang Y, Lei-Butters DC, Engelhardt JF . Unique characteristics of AAV1, 2, and 5 viral entry, intracellular trafficking, and nuclear import define transduction efficiency in HeLa cells. Hum Gene Ther 2011; 22: 1433–1444.
Nonnenmacher M, Weber T . Intracellular transport of recombinant adeno-associated virus vectors. Gene Therapy 2012; 19: 649–658.
Sonntag F, Bleker S, Leuchs B, Fischer R, Kleinschmidt JA . Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus. J Virol 2006; 80: 11040–11054.
Van Vliet K, Blouin V, Agbandje-McKenna M, Snyder RO . Proteolytic mapping of the adeno-associated virus capsid. Mol Ther 2006; 14: 809–821.
Lizee G, Aerts JL, Gonzales MI, Chinnasamy N, Morgan RA, Topalian SL . Real-time quantitative reverse transcriptase-polymerase chain reaction as a method for determining lentiviral vector titers and measuring transgene expression. Hum Gene Ther 2003; 14: 497–507.
Sambrook J, Fritsch EF, Maniatis T . Molecular Cloning a Laboratory Manual. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, 1989.
Acknowledgements
We thank Jim Wilson (University of Pennsylvania) for the AAV7, 8, 9 and rh10 capsids and Margot Latham (The Children’s Hospital at Westmead) for their assistance with manuscript preparation. Allison Dane was supported by an Australian National Health and Medical Research Council (NHMRC) Postgraduate Research Scholarship (477110) and Children’s Medical Research Institute PhD student stipend. The research work was supported by an NHMRC project grant (1008021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dane, A., Wowro, S., Cunningham, S. et al. Comparison of gene transfer to the murine liver following intraperitoneal and intraportal delivery of hepatotropic AAV pseudo-serotypes. Gene Ther 20, 460–464 (2013). https://doi.org/10.1038/gt.2012.67
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2012.67
Keywords
This article is cited by
-
The mitochondrial fusion protein OPA1 is dispensable in the liver and its absence induces mitohormesis to protect liver from drug-induced injury
Nature Communications (2023)
-
Integrated vector genomes may contribute to long-term expression in primate liver after AAV administration
Nature Biotechnology (2023)
-
Adeno-associated virus-based caveolin-1 delivery via different routes for the prevention of cholesterol gallstone formation
Lipids in Health and Disease (2022)
-
Vector engineering, strategies and targets in cancer gene therapy
Cancer Gene Therapy (2022)
-
Gene therapy in a murine model of chronic eosinophilic leukemia-not otherwise specified (CEL-NOS)
Leukemia (2022)