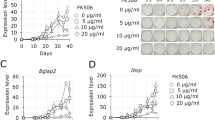
Abstract
Fibrodysplasia ossificans progressiva (FOP) is an autosomal dominant congenital disorder characterized by progressive heterotopic bone formation. Currently, no definitive treatment exists for FOP. The activin receptor type IA / activin-like kinase 2 (ACVR1/ALK2) gene has been identified as the responsible gene for FOP, and disease-associated ALK2 mutations have been found. Chemical inhibitors to the pathogenic ALK2 receptors are considered possible medical agents for FOP, but their adverse effects on normal ALK2 and other receptors cannot be excluded. Here we describe another treatment strategy for FOP using allele-specific RNA interference (ASP-RNAi), and show modified small interfering RNAs (siRNAs) conferring allele-specific silencing against disease-causing ALK2 mutants found in FOP, without affecting normal ALK2 allele. Thus, the siRNAs presented here may become novel therapeutic agents for FOP, and their induced ASP-RNAi may pave the way for the achievement of radical treatment of FOP and/or for the relief of its severe symptoms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Shore EM, Kaplan FS . Role of altered signal transduction in heterotopic ossification and fibrodysplasia ossificans progressiva. Curr Osteoporos Rep 2011; 9: 83–88.
Katagiri T . Heterotopic bone formation induced by bone morphogenesis protein signaling: fibrodysplasia ossificans progressiva. J Oral Biosci 2010; 52: 33–41.
Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet 2006; 38: 525–527.
Furuya H, Ikezoe K, Wang L, Ohyagi Y, Motomura K, Fujii N et al. A unique case of fibrodysplasia ossificans progressiva with an ACVR1 mutation, G356D, other than the common mutation (R206H). Am J Med Genet A 2008; 146A: 459–463.
Fukuda T, Kohda M, Kanomata K, Nojima J, Nakamura A, Kamizono J et al. Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem 2009; 284: 7149–7156.
Shen Q, Little SC, Xu M, Haupt J, Ast C, Katagiri T et al. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest 2009; 119: 3462–3472.
Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol 2010; 5: 245–253.
Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML et al. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med 2008; 14: 1363–1369.
Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 2008; 4: 33–41.
Kaplan J, Kaplan F, Shore EM . Development of allele-specific RNAi as a therapeutic strategy for fibrodysplasia ossificans progressiva. J Bone Miner Res 2009 (Supplement-1) (http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=59e0e08f-6568-4b1b-80d5-fcc93765f2a5).
Hohjoh H . Allele-specific silencing by RNA interference. Methods Mol Biol 2010; 623: 67–79.
Ohnishi Y, Tamura Y, Yoshida M, Tokunaga K, Hohjoh H . Enhancement of allele discrimination by introduction of nucleotide mismatches into siRNA in allele-specific gene silencing by RNAi. PLoS One 2008; 3: e2248.
Ohnishi Y, Tokunaga K, Kaneko K, Hohjoh H . Assessment of allele-specific gene silencing by RNA interference with mutant and wild-type reporter alleles. J RNAi Gene Silencing 2006; 2: 154–160.
Takahashi M, Watanabe S, Murata M, Furuya H, Kanazawa I, Wada K et al. Tailor-made RNAi knockdown against triplet repeat disease-causing alleles. Proc Natl Acad Sci USA 2010; 107: 21731–21736.
Katagiri T, Imada M, Yanai T, Suda T, Takahashi N, Kamijo R . Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells 2002; 7: 949–960.
Kaplan FS, Fiori J, LS DLP, Ahn J, Billings PC, Shore EM . Dysregulation of the BMP-4 signaling pathway in fibrodysplasia ossificans progressiva. Ann N Y Acad Sci 2006; 1068: 54–65.
Fukuda T, Kanomata K, Nojima J, Kokabu S, Akita M, Ikebuchi K et al. A unique mutation of ALK2, G356D, found in a patient with fibrodysplasia ossificans progressiva is a moderately activated BMP type I receptor. Biochem Biophys Res Commun 2008; 377: 905–909.
Podesta JE, Kostarelos K . Chapter 17 – Engineering cationic liposome siRNA complexes for in vitro and in vivo delivery. Methods Enzymol 2009; 464: 343–354.
Hanai K, Takeshita F, Honma K, Nagahara S, Maeda M, Minakuchi Y et al. Atelocollagen-mediated systemic DDS for nucleic acid medicines. Ann N Y Acad Sci 2006; 1082: 9–17.
Ochiya T, Takahama Y, Nagahara S, Sumita Y, Hisada A, Itoh H et al. New delivery system for plasmid DNA in vivo using atelocollagen as a carrier material: the Minipellet. Nat Med 1999; 5: 707–710.
Acknowledgements
This work was supported by research grants from the Ministry of Health, Labour and Welfare of Japan and from the National Hospital Organization, and also by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and by Grant-in-Aid for Young Scientists (Start-up).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Takahashi, M., Katagiri, T., Furuya, H. et al. Disease-causing allele-specific silencing against the ALK2 mutants, R206H and G356D, in fibrodysplasia ossificans progressiva. Gene Ther 19, 781–785 (2012). https://doi.org/10.1038/gt.2011.193
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.193
Keywords
This article is cited by
-
Macrophages in heterotopic ossification: from mechanisms to therapy
npj Regenerative Medicine (2021)
-
The biological function of type I receptors of bone morphogenetic protein in bone
Bone Research (2016)
-
A novel measurement of allele discrimination for assessment of allele-specific silencing by RNA interference
Molecular Biology Reports (2014)
-
Silencing the FOP gene
Gene Therapy (2012)