Abstract
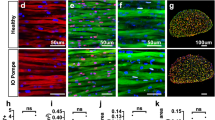
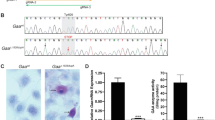
Glycogen storage disease type II (Pompe disease; MIM 232300) stems from the inherited deficiency of acid-α-glucosidase (GAA; acid maltase; EC 3.2.1.20), which primarily involves cardiac and skeletal muscles. We hypothesized that hydrostatic isolated limb perfusion (ILP) administration of an adeno-associated virus (AAV) vector containing a muscle-specific promoter could achieve relatively higher transgene expression in the hindlimb muscles of GAA-knockout (GAA-KO) mice, in comparison with intravenous (IV) administration. ILP administration of AAV2/8 vectors encoding alkaline phosphatase or human GAA-transduced skeletal muscles of the hindlimb widely, despite the relatively low number of vector particles administered (1 × 1011), and IV administration of an equivalent vector dose failed to transduce skeletal muscle detectably. Similarly, ILP administration of fewer vector particles of the AAV2/9 vector encoding human GAA (3 × 1010) transduced skeletal muscles of the hindlimb widely and significantly reduced glycogen content to, in comparison with IV administration. The only advantage for IV administration was moderately high-level transduction of cardiac muscle, which demonstrated compellingly that ILP administration sequestered vector particles within the perfused limb. Reduction of glycogen storage in the extensor digitorum longus demonstrated the potential advantage of ILP-mediated delivery of AAV vectors in Pompe disease, because type II myofibers are resistant to enzyme replacement therapy. Thus, ILP will enhance AAV transduction of multiple skeletal muscles while reducing the required dosages in terms of vector particle numbers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hirschhorn R, Reuser AJJ . Glycogen storage disease type II: acid a-glucosidase (acid maltase) deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds). The Metabolic and Molecular Basis for Inherited Disease. McGraw-Hill: New York, 2001, pp 3389–3420.
Hoefsloot LH, Hoogeveen-Westerveld M, Reuser AJ, Oostra BA . Characterization of the human lysosomal alpha-glucosidase gene. Biochem J 1990; 1: 493–497.
Wisselaar HA, Kroos MA, Hermans MMP, Vanbeeumen J, Reuser AJJ . Structural and functional-changes of lysosomal acid alpha-glucosidase during intracellular-transport and maturation. J Biol Chem 1993; 268: 2223–2231.
Bijvoet AG, Van Hirtum H, Kroos MA, Van de Kamp EH, Schoneveld O, Visser P et al. Human acid alpha-glucosidase from rabbit milk has therapeutic effect in mice with glycogen storage disease type II. Hum Mol Genet 1999; 8: 2145–2153.
Raben N, Danon M, Gilbert AL, Dwivedi S, Collins B, Thurberg BL et al. Enzyme replacement therapy in the mouse model of Pompe disease. Mol Genet Metab 2003; 80: 159–169.
Amalfitano A, Bengur AR, Morse RP, Majure JM, Case LE, Veerling DL et al. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet Med 2001; 3: 132–138.
Van den Hout JMP, Kamphoven JHJ, Winkel LPF, Arts WFM, De Klerk JBC, Loonen MCB et al. Long term intravenous treatment of Pompe disease with recombinant human alpha-glucosidase from milk. Pediatrics 2004; 113: E448–E457.
Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL et al. Recombinant human acid {alpha}-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology 2007; 68: 99–109.
Kishnani PS, Goldenberg PC, Dearmey SL, Heller J, Benjamin D, Young S et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab 2010; 99: 26–36.
Franco LM, Sun B, Yang X, Bird A, Zhang H, Schneider A et al. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther 2005; 12: 876–884.
Ziegler RJ, Bercury SD, Fidler J, Zhao MA, Foley J, Taksir TV et al. Ability of adeno-associated virus serotype 8-mediated hepatic expression of acid alpha-glucosidase to correct the biochemical and motor function deficits of presymptomatic and symptomatic Pompe mice. Hum Gene Ther 2008; 19: 609–621.
Sun B, Zhang H, Benjamin Jr DK, Brown T, Bird A, Young SP et al. Enhanced efficacy of an AAV vector encoding chimeric, highly secreted acid alpha-glucosidase in glycogen storage disease type II. Mol Ther 2006; 14: 822–830.
Sun B, Zhang H, Franco LM, Brown T, Bird A, Schneider A et al. Correction of glycogen storage disease type II by an adeno-associated virus vector containing a muscle-specific promoter. Mol Ther 2005; 11: 889–898.
Sun B, Young SP, Li P, Di C, Brown T, Salva MZ et al. Correction of multiple striated muscles in murine Pompe disease through adeno-associated virus-mediated gene therapy. Mol Ther 2008; 16: 1366–1371.
Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med 2004; 10: 828–834.
Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med 2006; 12: 342–347.
Hagstrom JE, Hegge J, Zhang G, Noble M, Budker V, Lewis DL et al. A facile nonviral method for delivering genes and siRNAs to skeletal muscle of mammalian limbs. Mol Ther 2004; 10: 386–398.
Su LT, Gopal K, Wang Z, Yin X, Nelson A, Kozyak BW et al. Uniform scale-independent gene transfer to striated muscle after transvenular extravasation of vector. Circulation 2005; 112: 1780–1788.
Qiao C, Li J, Zheng H, Bogan J, Li J, Yuan Z et al. Hydrodynamic limb vein injection of AAV8 canine myostatin propeptide gene in normal dogs enhances muscle growth. Hum Gene Ther 2009; 20: 1–10.
Salva MZ, Himeda CL, Tai PW, Nishiuchi E, Gregorevic P, Allen JM et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol Ther 2007; 15: 320–329.
Gregorevic P, Schultz BR, Allen JM, Halldorson JB, Blankinship MJ, Meznarich NA et al. Evaluation of vascular delivery methodologies to enhance rAAV6-mediated gene transfer to canine striated musculature. Mol Ther 2009; 17: 1427–1433.
Toumi H, Hegge J, Subbotin V, Noble M, Herweijer H, Best TM et al. Rapid intravascular injection into limb skeletal muscle: a damage assessment study. Mol Ther 2006; 13: 229–236.
Vigen KK, Hegge JO, Zhang G, Mukherjee R, Braun S, Grist TM et al. Magnetic resonance imaging-monitored plasmid DNA delivery in primate limb muscle. Hum Gene Ther 2007; 18: 257–268.
Hegge JO, Wooddell CI, Zhang G, Hagstrom JE, Braun S, Huss T et al. Evaluation of hydrodynamic limb vein injections in non-human primates. Hum Gene Ther 2010; 21: 829–842.
Fukuda T, Roberts A, Ahearn M, Zaal K, Ralston E, Plotz PH et al. Autophagy and lysosomes in Pompe disease. Autophagy 2006; 2: 318–320.
Haida N, Fowler Jr WM, Abresch RT, Larson DB, Sharman RB, Taylor RG et al. Effect of hind-limb suspension on young and adult skeletal muscle. I. Normal mice. Exp Neurol 1989; 103: 68–76.
Wortmann RL, DiMauro S . Differentiating idiopathic inflammatory myopathies from metabolic myopathies. Rheum Dis Clin N Am 2002; 28: 759–778.
Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM . Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA 2002; 99: 11854–11859.
Raben N, Nagaraju K, Lee E, Kessler P, Byrne B, Lee L et al. Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem 1998; 273: 19086–19092.
Amalfitano A, McVie-Wylie AJ, Hu H, Dawson TL, Raben N, Plotz P et al. Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-alpha-glucosidase. Proc Natl Acad Sci USA 1999; 96: 8861–8866.
Sun B, Zhang H, Franco LM, Young SP, Schneider A, Bird A et al. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol Ther 2005; 11: 57–65.
Acknowledgements
This work was supported by NIH Grant R01 HL081122 from the National Heart, Lung and Blood Institute. BS was supported by the Muscular Dystrophy Association. GAA-KO mice were provided courtesy of Dr Nina Raben at the National Institutes of Health (Bethesda, MD, USA). The AAV8 and AAV9 packaging plasmids were provided courtesy of Dr James M Wilson at the University of Pennsylvania (Philadelphia, PA, USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sun, B., Li, S., Bird, A. et al. Hydrostatic isolated limb perfusion with adeno-associated virus vectors enhances correction of skeletal muscle in Pompe disease. Gene Ther 17, 1500–1505 (2010). https://doi.org/10.1038/gt.2010.109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2010.109