Abstract
Purpose
To assess qualitative corneal changes and penetration of pulsed and continuous light accelerated crosslinking by in vivo confocal microscopy and corneal OCT.
Methods
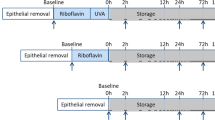
A total of 20 patients affected from progressive keratoconus were enrolled in the study. Ten eyes of 10 patients underwent an epithelium-off pulsed-light accelerated corneal collagen crosslinking (PL-ACXL) by the KXL UV-A source (Avedro Inc.) with 8 min (1 s on/1 s off) of UV-A exposure at 30 mW/cm2 and energy dose of 7.2 J/cm2; 10 eyes of 10 patients underwent an epithelium-off continuous-light accelerated corneal collagen crosslinking (CL-ACXL) at 30 mW/cm2 for 4 min. Riboflavin 0.1% dextran-free plus hydroxyl-propyl-methylcellulose solution (VibeX Rapid, Avedro Inc.) was used for a 10-min corneal soaking. Treated eyes were examined by in vivo scanning laser confocal analysis and spectral anterior segment OCT at 1, 3, and 6 months.
Results
Epithelial stratification and nerves regeneration improved in time, being complete at month 6 in both groups without endothelial damage. Keratocyte apoptosis in PL-ACXL was estimated at a mean depth of ∼200 μm, whereas an uneven demarcation line was detectable by confocal microscopy at a mean depth of 160 μm in CL-ACXL.
Conclusion
In vivo confocal microscopy and corneal OCT allowed a precise qualitative analysis of the cornea after epithelium-off PL-ACXL and CL-ACXL treatments. Apoptotic effect was higher in pulsed than in continuous light treatments, exceeding 200 μm in corneal stroma. According to different morphological data, the clinical efficacy of ACXL needs to be determined in a long-term follow-up and large cohort of patients.
Similar content being viewed by others
Introduction
Riboflavin UV-A-induced corneal collagen crosslinking (CXL) represents a relatively new procedure available for the conservative treatment of progressive keratoconus1, 2 and secondary corneal ectasia3 because of its capacity in increasing biomechanical corneal resistance4, 5 and intrinsic anticollagenase activity.6 The physicochemical basis of crosslinking lies in the photo-dynamic type I–II reactions7 induced by the interaction between 0.1% riboflavin molecules absorbed in corneal tissue and UV-A rays delivered at 3 mW/cm2 for 30 min (5.4 J/cm2 energy dose) releasing reactive oxygen species (ROS) that mediated crosslink formation between and within collagen fibers.8, 9 Conventional epithelium-off crosslinking (CXL) demonstrated its safety and long-term efficacy in stabilizing progressive keratoconus and secondary ectasias in different clinical trials.10, 11, 12, 13, 14 On the other hand, the procedure is time consuming, lasting from 40 min to 1 h.15 The Bunsen–Roscoe’s law of reciprocity16, 17, 18 theoretically demonstrated that the photochemical process behind crosslinking depends on the absorbed UV-A energy and its biological effect is proportional to the total energy dose delivered in the tissue.16, 17, 18 According to this concept, it is theoretically possible to deliver the same energy dose ensuring a proportional biological effect by setting different UV-A powers and exposure times in order to accelerate and shorten the crosslinking procedure in accelerated crosslinking (ACXL) modality.17, 18, 19 According to photochemical crosslinking studies based on kinetics model7 the UV-A illumination caused a rapid depletion of oxygen in a riboflavin-soaked cornea and turning the UV light off led to replenishment of the oxygen to its original level within 3 to 4 min.7 Krueger et al20 and Herekar21 have also observed a rapid oxygen depletion during corneal crosslinking with riboflavin and concluded that the ROS and, specifically, singlet oxygen are the predominant CXL reaction drivers. Under aerobic conditions, which are present during the first 10 to 15 s of UV-A exposure, sensitized photooxidation of the substrate (proteoglycan core proteins22 and collagen in the corneal matrix) occurs mainly by its reaction with photochemically generated ROS, such as singlet molecular oxygen. This is consistent with a type II photochemical mechanism. After the first 10 to 15 s, oxygen becomes totally depleted and the reaction between the substrate and riboflavin becomes consistent with a predominantly type I photochemical mechanism.7 Pulsing the UV light during crosslinking treatment theoretically restarts the photodynamic type II reaction achieving an additional oxygen concentration allowing more singlet oxygen release for crosslinking of collagen molecules. We report an in vivo scanning laser confocal microscopy and anterior chamber spectral domain OCT micro-morphological analysis in a series of 20 patients with progressive keratoconus investigating the qualitative corneal changes and estimating the penetration of pulsed light accelerated crosslinking (PL-ACXL) and continuous light accelerated crosslinking (CL-ACXL).
Materials and Methods
After Institutional Review Board of the Siena University Hospital approval and specific informed consent subscription, 20 patients affected from progressive keratoconus were enrolled in the study. They were divided into two treatment groups: 10 eyes of 10 patients (pulsed light treatments), age between 13 and 26 years (mean 21.5 years), underwent an epithelium-off PL-ACXL by the KXL I UV-A source (Avedro Inc., Waltham, MS, USA) with 8 min (1 s on/ 1 s off) of UV A exposure at 30 mW/cm2 with an energy dose of 7.2 J/cm2; 10 eyes of 10 patients (continuous light treatments), age between 11 and 24 years (mean 18,5 years), underwent an epithelium-off CLA-XL with the same instrument, UV-A power setting at 30 mW/cm2 for 4 min of continuous UV-A light exposure and energy dose23 of 7.2 J/cm2. After epithelium removal by a blunt metal spatula in a 9-mm circle, the cornea was imbibed for 10 min,23, 24, 25 administering 3–5 drops of dextran-free riboflavin 0.1% with hydroxyl-propyl-methylcellulose (VibeX Rapid, Avedro Inc.)23, 24 at 1 min interval, covering all corneal surface, including the limbus. Before starting UV-A irradiation, corneal thickness was checked by intraoperative optical OCT corneal pachymetry to ensure a constant minimum stromal thickness of 350 μm.26 The riboflavin solution was administered along the UV-A exposure at 2.5 min intervals. At the end of the procedures, treated eyes were dressed by a soft contact lens bandage for 3 days and medicated with ciprofloxacin eye drops, diclofenac, and sodium hyaluronate eye drops 4 times/day. Postoperative in vivo qualitative analysis of corneal changes was assessed by the HRT II system (Rostock Cornea Module, Heidelberg, Germany) in vivo scanning laser confocal microscopy (IVCM) and spectral domain (SD) corneal OCT (Cirrus, Zeiss Meditec, Jena, Germany), estimating treatment penetration.
Inclusion criteria
The parameters we considered to establish keratoconus progression and inclusion criteria for each group were: worsening of UCVA/BSCVA >0.5 decimal equivalents, increase of Sph/Cyl >0.5 D, increase of topographic symmetry index SAI/SI >0.5 D, increase of simulated maximum K reading >1 D, reduction of the thinnest point at corneal OCT optical pachymetry ≥10 μm, clear cornea at biomicroscopic examination, and absence of reticular dark striations at confocal laser microscopy in vivo. We considered relevant for the inclusion in the treatment protocol the variation of at least three of the parameters listed above (one clinical plus two instrumental) in the past 6 months of observation. All patients had a corneal thinnest point over 400 μm without epithelium. The following examinations were performed before and after the operation: in vivo scanning laser confocal microscopy (HRT II, Rostock Cornea Module) and anterior segment OCT analysis (Visante OCT, Zeiss Meditec) to assess qualitative ACXL-induced corneal changes and treatment penetration.
Results
PLA-XL
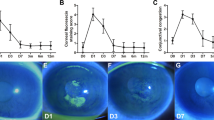
All eyes re-epithelialized by 3 days of therapeutic soft contact lens bandage. Epithelial stratification improved in time, being complete at month 3. Subepithelial and anterior stromal nerves disappeared immediately after treatment. Nerve regeneration started 1 month after treatment being complete after 6 months. Anterior stromal tissue presented a high reflectivity after pulsed light ACXL with keratocytes loss (apoptosis hence photonecrosis) until ∼200 μm of depth (meanly at 198.43 μm, range 188–212 μm) and classical spongy or lacunar edema as previously demonstrated by us27, 28 in conventional epithelium-off CXL was evident until month 3, gradually disappearing thereafter. Keratocytes repopulation started 1 month after treatment, increasing at month 3, and being complete at postoperative month 6. An uneven demarcation line was determined by confocal analysis at a mean depth of 200 μm (range 190–210 μm) measured from epithelial surface (Figure 1a). Confocal data of increased stromal reflectivity and demarcation line (defined by corneal edema and keratocytes apoptosis with changes in stromal reflectivity) were established by anterior chamber OCT at a mean depth of 180 μm (range 180–210 μm; Figure 1b). The demarcation line was also clinically evident after pulsed light treatment at slit lamp examination (Figure 1c). No postoperative endothelial damage or cell loss was detected after pulsed light ACXL.
Pulsed light accelerated crosslinking (PL-ACXL). Confocal scan reveals keratocytes apoptosis (K. Apo), lacunar corneal edema (C.E.), and activated keratocytes nuclei (A.K.N.) with a mean penetration of 200 μm (a); spectral domain corneal OCT performed before removing therapeutic contact lens after treatment (T.C.L.) reveals an increased reflectivity of the anterior mid-stroma after treatment with an inhomogeneous demarcation line (D.L.) at 180 μm of depth (b); slit lamp examination at first postoperative month reveals a visible demarcation line (D.L.) (c).
CLA-XL
Epithelial regrowth and nerve regeneration were similar with those observed in pulsed light epithelium-off treatments. Whereas epithelium regenerates rapidly into 3 days, neural flocculation was detectable 1 month after treatment. Stromal healing revealed a lower reflectivity than in pulsed light treatments. An uneven demarcation line is detectable by confocal microscopy at a mean depth of 160 μm (range 150–180 μm; Figure 2a). Demarcation line depth was confirmed by corneal OCT at a mean depth of 160 μm (range 140–180 μm; Figure 2b), also visible at slit lamp examination (Figure 2c). No endothelial damage was detectable in the postoperative evaluation.
Continuous light accelerated crosslinking (CL-ACXL) confocal scan reveals keratocytes apoptosis (K. Apo), lacunar corneal edema (C.E.), and activated keratocytes nuclei (A.K.N.) with a mean penetration of 160 μm (a); spectral domain corneal OCT performed after therapeutic contact lens removal reveals an increased reflectivity of the anterior mid-stroma after treatment with an inhomogeneous demarcation line (D.L.) at 160 μm of depth (b); slit lamp examination at first postoperative month reveals a visible demarcation line (D.L.) (c).
Preoperative mean endothelial cell density was 2450 cells/mm2 (range, 2082–3026 cells/mm2). In the continuous light ACXL treatments, it was 2672 cells/mm2 (range 2459–3016 cells/mm2). Postoperative endothelial cell count at month 6 was 2386 cells/mm2 on average (range 2112–2989 cells/mm2) in the CL-ACXL group and 2597 cells/mm2 (range 2416–3127 cells/mm2) in the PL-ACXL group.
Conclusions
In vivo scanning laser confocal microscopy allowed a precise qualitative analysis of the cornea after epithelium-off PL-ACXL and CL-ACXL treatments. Corneal changes included time-dependent epithelial stratification, nerves disappearance and regeneration, keratocytes loss with progressive repopulation, and stromal healing. Corneal OCT scans provided an insight in the identification of demarcation lines estimating approximately treatment’s penetration. PL-ACXL showed an apoptotic effect meanly at 200 μm of stromal depth (range 190–240 μm), whereas CL-ACXL revealed a penetration of 160 μm on average (range 150–200 μm), both at confocal and corneal OCT analysis, that appeared inferior (approximately −40 μm) to pulsed light. These findings were slightly better than those recently reported in literature,29 probably because of the higher energy dose used in these treatments (7.2 J/cm2 instead of 5.4 J/cm2) and pulsed light modality. Indeed, pulsing the UV-A light inducing an intraoperative oxygen reuptake while prolonging treatment time at 8 min may influence a deeper penetration of oxidative damage.30
The clinical aspect of the corneas after ACXL was good in both groups after therapeutic soft contact lens removal without complications. A demarcation line was detectable at slit lamp examination after therapeutic soft contact lens removal, as shown in Figures 1c and 2c. ACXL with pulsed (PL-ACXL) and continuous UV-A light illumination (CL-ACXL) reached the anterior mid-part of the corneal stroma until a maximum of 240 μm and 200 μm of depth, respectively, measured from epithelium surface. This aspect assumes a physicochemical relevance because, as reported in literature,31 the most important biomechanical effect related to crosslinking is concentrated in the anterior mid-stroma. To date, we do not know exactly the optimal interactions between UV-A energy, riboflavin concentration, and exposure time in order to obtain the maximum crosslinking effect ensuring a long-lasting (possibly a definitive) keratoconus biomechanical stability and the better functional outcome, even if the necessity to improve and shorten the procedure are highly desirable. Conventional epithelium-off CXL procedure (Riboflavin 0.1% plus dextran 20%, UV-A 3 mW/cm2=5.4 J/cm2 for 30 min) was proved to be safe and efficacious in the conservative treatment of early-stage progressive keratoconus. Anyway, in our preliminary experience, the ACXL with epithelium removal demonstrated its safety for endothelium in both pulsed and continuous light treatment modalities, shortening the CXL procedure time under 20 min, and being well tolerated by patients. Pulsed light treatment seems slightly more capable to penetrate deeper in the corneal stroma as compared with continuous light treatment. Pulsed and continuous light ACXL represent evolving crosslinking procedures in order to achieve keratoconus stabilization in a short treatment time. The efficacy of these techniques needs to be determined in the mid- to long-term follow-up and in a large cohort of patients.

References
Wollensak G, Spoerl E, Seiler Th . Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophtalmol 2003; 135 (5): 620–627.
Caporossi A, Baiocchi S, Mazzotta C, Traversi C, Caporossi T . Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A-induced crosslinking of corneal collagen: preliminary refractive results in an Italian study. J Cataract Refract Surg 2006; 32 (5): 837–845.
Hafezi F, Kanellopoulos J, Wiltfang R, Seiler T . Corneal collagen crosslinking with riboflavin and ultraviolet A to treat induced keratectasia after laser in situ keratomileusis. J Cataract Refract Surg 2007; 33 (12): 2035–2040.
Wollensak G, Spoerl E, Seiler T . Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced crosslinking. J Cataract Refract Surg 2003; 29 (9): 1780–1785.
Wollensak G, Wilsch M, Spoerl E, Seiler T . Collagen fiber diameter in the rabbit cornea after collagen crosslinking by riboflavin/UVA. Cornea 2004; 23 (5): 503–507.
Spoerl E, Wollensak G, Seiler T . Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res 2004; 29 (1): 35–40.
Kamaev P, Friedman MD, Sherr E, Muller D . Photochemical kinetics of corneal crosslinking with riboflavin. Invest Ophthalmol Vis Sci. 2012; 53 (4): 2360–2367.
Spoerl E, Huhle M, Seiler T . The Induction of cross links in corneal tissue. Exp Eye Res 1998; 66: 97–103.
Spoerl E, Seiler T . Techniques for stiffening the cornea. J Refract Surg 1999; 15 (6): 711–713.
Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE . Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: Long-term results. J Cataract Refract Surg 2008; 34 (5): 796–801.
Caporossi A, Mazzotta C, Baiocchi S, Caporossi T . Long-term results of riboflavin ultraviolet A corneal collagen crosslinking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol 2010; 149 (4): 585–593.
Wittig-Silva C, Whiting M, Lamoureux E, Lindsay RG, Sullivan LJ, Snibson GR . A randomized controlled trial of corneal collagen crosslinking in progressive keratoconus: preliminary results. J Refract Surg 2008; 24 (7): S720–S725.
Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, Denaro R . Age-related long-term functional results after riboflavin UV A corneal crosslinking. J Ophthalmol 2011; 2011: 608041.
Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, Denaro R, Balestrazzi A . Riboflavin-UVA-induced corneal collagen crosslinking in pediatric patients. Cornea 2012; 31 (3): 227–231.
Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T . Safety of UVA-riboflavin crosslinking of the cornea. Cornea 2007; 26 (4): 385–389.
Brindley Gs . The Bunsen-Roscoe law for the human eye at very short durations. J Physiol 1952; 118 (1): 135–139.
Schumacher S, Oeftiger L, Mrochen M . Equivalence of biomechanical changes induced by rapid and standard corneal crosslinking, using riboflavin and ultraviolet radiation. Invest Ophthalmol Vis Sci 2011; 52 (12): 9048–9052.
Wernli J, Schumacher S, Spoerl E, Mrochen M . The efficacy of corneal crosslinking shows a sudden decrease with very high intensity UV light and short treatment time. Invest Ophthalmol Vis Sci 2013; 54 (2): 1176–1180.
Celik HU, Alagöz N, Yildirim Y, Agca A, Marshall J, Demirok A et al. Accelerated corneal crosslinking concurrent with laser in situ keratomileusis. J Cataract Refract Surg 2012; 38 (8): 1424–1431.
Krueger RR, Spoerl E, Herekar S . Rapid vs standard collagen CXL with equivalent energy dosing. In: Proceedings of the Third International Congress of Corneal Collagen Crosslinking 2007 Zurich, Switzerland.
Herekar SV inventor; Method for equi-dosed time fractionated pulsed UVA irradiation of collagen/riboflavin mixtures for ocular structural augmentation. US patent US2009/0149923A1 11 June 2009.
Wollensak G, Spörl E, Mazzotta C, Kalinski T, Sel S . Interlamellar cohesion after corneal crosslinking using riboflavin and ultraviolet A light. Br J Ophthalmol 2011; 95 (6): 876–880.
Mazzotta C, Paradiso AL, Baiocchi S, Caragiuli S, Caporossi A . Qualitative investigation of corneal changes after accelerated corneal collagen cross-linking (A-CXL) by in vivo confocal microscopy and corneal OCT. J Clin Exp Ophthalmol 2013; 4: 313.
Mazzotta C, Baiocchi S, Caporossi T, Caragiuli S, Paradiso AL, Caporossi A . Riboflavin 0.1% (VibeX) for the treatment of keratoconus. Expert Opin Orphan Drugs 2013; 1 (3): 235–240.
Baiocchi S, Mazzotta C, Cerretani D, Caporossi T, Caporossi A . Corneal crosslinking: riboflavin concentration in corneal stroma exposed with and without epithelium. J Cataract Refract Surg. 2009; 35 (5): 893–899.
Mazzotta C, Caragiuli S . Intraoperative corneal thickness measurement by optical coherence tomography in keratoconic patients undergoing corneal collagen cross-linking. Am J Ophthalmol 2014; 157 (6): 1156–1162.
Mazzotta C, Balestrazzi A, Traversi C, Baiocchi S, Caporossi T, Tommasi C et al. Treatment of progressive keratoconus by riboflavin-UVA-induced crosslinking of corneal collagen: ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea 2007; 26 (4): 390–397.
Mazzotta C, Traversi C, Baiocchi S, Caporossi O, Bovone C, Sparano MC et al. Corneal healing after riboflavin ultraviolet-A collagen crosslinking determined by confocal laser scanning microscopy in vivo: early and late modifications. Am J Ophthalmol 2008; 146 (4): 527–533.
Touboul D, Efron N, Smadja D, Praud D, Malet F, Colin J . Corneal confocal microscopy following conventional, transepithelial, and accelerated corneal collagen crosslinking procedures for keratoconus. J Refract Surg 2012; 28 (11): 769–776.
Merwald H, Klosner G, Kokesch C, Der-Petrossian M, Hönigsmann H, Trautinger F . UVA-induced oxidative damage and cytotoxicity depend on the mode of exposure. J Photochem Photobiol B 2005; 79 (3): 197–207.
Schumacher S, Mrochen M, Wernli J, Bueeler M, Seiler T . Optimization model for UV-riboflavin corneal crosslinking. Invest Ophthalmol Vis Sci 2012; 53 (2): 762–769.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mazzotta, C., Traversi, C., Caragiuli, S. et al. Pulsed vs continuous light accelerated corneal collagen crosslinking: in vivo qualitative investigation by confocal microscopy and corneal OCT. Eye 28, 1179–1183 (2014). https://doi.org/10.1038/eye.2014.163
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.163
This article is cited by
-
The effect of accelerated pulsed high-fluence corneal cross-linking on corneal endothelium; a prospective specular microscopy study
BMC Ophthalmology (2023)
-
Indikationsstellung zum Crosslinking und klinische Ergebnisse neuer kornealer Crosslinking-Techniken
Der Ophthalmologe (2022)
-
Long term results of accelerated 9 mW corneal crosslinking for early progressive keratoconus: the Siena Eye-Cross Study 2
Eye and Vision (2021)
-
Singlet oxygen formation during accelerated and hyperaccelerated corneal cross-linking: in vitro study
Eye (2021)
-
New perspectives in keratoconus treatment: an update on iontophoresis-assisted corneal collagen crosslinking
International Ophthalmology (2021)