Abstract
Purpose
To report the clinicopathologic features in a series of patients after ineffective glaucoma surgery with gold micro shunts (GMS) 2 years after the procedure.
Methods
This was an interventional case series study including two cases of GMS and two of GMS+ removal. Each specimen was sectioned into three portions: proximal, middle, and distal, and embedded into paraffin blocks, cut into 3 μm sections and stained with hematoxylin and eosin and Masson’s trichrome. In the case of inflammatory infiltrations a reaction with an LCA (CD45) monoclonal antibody was performed.
Results
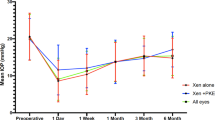
Mean IOP before GMS removal was 28.8±4.3 mm Hg, and the patients were administered 2.3±0.5 anti-glaucoma drugs. The progression of changes in the visual field was observed in all cases. In three cases different grade intensification of corneal decompression was observed. Colonization of the connective tissue was found in the channels and around the microimplant in all cases. In two cases infiltration was detected from giant polynuclear and mononuclear cells.
Conclusions
Connective tissue colonization was the cause of GMS obstruction. This can be a non-inflammatory process, but it may also result from chronic inflammation occurring in the suprachoroidal space.
Similar content being viewed by others
Introduction
Hydrostatic pressure difference between the anterior chamber and the suprachoroidal space is the basis for surgical use of the unconventional outflow pathway.1 Implants enhancing the unconventional outflow pathway have already undergone specific evolution through GMS (SOLX Inc., Occulogix, Waltham, MA, USA) used via an ab externo approach to CyPassMicro-Stent (Transcend Medical) the implantation of which is performed from the anterior chamber to the suprachoroidal space.2, 3 However, it seems that the physiology of suprachoroidal space has not been well recognized yet as regards the possibility of IOP regulation and the reaction to the presence of a foreign body in the long-term follow-up.
Case reports
There were four patients aged 70.2±15.5 years with POAG. Mean IOP before GMS implantation was 22.5±3.5 mm Hg, and the patients used 2.5±0.6 (median: Me−2.5) medications. The mean IOP before GMS removal was 28.8±4.3 mm Hg (P=0.002), and the patients used 2.3±0.5 (Me−2.0) medications (P=0.361). The mean time for the implant removal was 29.8±8.3 months.
Case 1
A 90-year-old man with GMS removed from the right eye 29 months after implantation. IOP was 25.0 mm Hg (Ganfort), and progression in the visual field was observed (MD=−17.91). The central corneal thickness (CCT) increased from 480 to 568 μm and the corneal decompensation was detected despite the lack of GMS contact with the endothelium (OCT Visante). In the middle portion of GMS the presence of collagen-rich connective capsule-like reaction about 100-μm thick was found (Figure 1). The presence of cell-rich collagen-poor connective tissue was also found between the plates. There were also observed chronic inflammatory infiltration cells, polynuclear giant cells, and small blood vessels (Figure 2a).
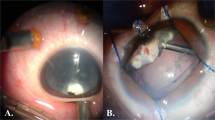
Macroscopic image of the removed microimplant (case 1). Colonization of the connective tissue around the microimplant. (a) Proximal portion; fragments of the connective tissue at the side of the sclera, (b–d) distal portion: connective tissue (at the choroidal side) blocking the channels of the microimplant (Cannon EF 100 mm f/2.8 L Macro IS USM).
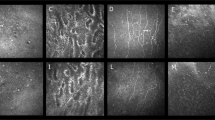
Microphotograph of the removed microimplant. (a) Case 1: in the middle portion the implant is surrounded by collagen-rich connective tissue capsule. A cluster of polynuclear giant cells present between the implant plates (asterisk). On the left side the giant cells combine with collagen-poor granulation tissue with numerous small blood vessels filling the remaining part of the microimplant channel. Below, within the sclera, is shown a visible focus of dispersed inflammatory infiltration (arrow) (H&E × 200). (b) Case 3: amorphous thickened protein masses between microimplant plates (H&E × 200). (c) Case 4: inflammatory infiltrations from lymphocytic cells between the GMS plates (H&E × 400). (d) Case 4: the membrane expression of LCA (CD45) in the infiltration from mononuclear cells (immunohistochemical staining with a anti-LCA antibody × 400).
Case 2
In the case of a 69-year-old woman GMS+ was removed from the left eye 21 months after surgery. Before removal, IOP was 24.0 mm Hg (Xalacom). Progression in the visual field (MD=−8.55) and corneal decompensation were observed. CCT increased from 534 to 676 μm despite the lack of GMS contact with the endothelium. In the middle portion of GMS the presence of collagen-rich connective capsule-like reaction was detected, but the capsule was thin (about 50 μm). In case no. 2 the presence of cell-rich collagen-poor connective tissue was found between the plates.
Case 3
The cause of GMS removal from a right eye of a 70-year-old man was the decrease of endothelium from 2579 to 1685 cells mm−2 despite the lack of contact with GMS, and progression in the visual field was observed (MD=−3.49). IOP was 22.0 mm Hg (Ganfort) 28 months after the surgery. In the middle and distal part the GMS was surrounded by collagen-rich connective tissue capsule. The proximal, middle and distal portion channels were partially filled with dense amorphous protein masses (Figure 2b).
Case 4
In a 52-year-old man, 41 months after primary surgery, GMS+ was removed from the right eye because of the lack of IOP control despite of the maximal antiglaucoma therapy (Duotrav, Azopt). There were observed an increase of IOP to 41.0 mm Hg and progression in the visual field (MD=−4.23). No contact of the implant with the endothelium was observed. Collagen-rich connective tissue in the implant channels and dispersed chronic inflammatory infiltrations both around and inside the implant were detected. Lymphocytic infiltration (LCA+) was accompanied with clusters of numerous polynuclear giant cells (Figure 2c and d).
Discussion
GMS is one of the most modern implants improving the unconventional pathway outflow. Despite encouraging initial clinical reports, in the medium-term follow-up the IOP reduction decreased, whereas the number of applied anti-glaucoma medications increased.4 It was suggested that the formation of a fibrotic capsule around the device was responsible for the failure of the GMS surgery.4, 5, 6 We found fibroblasts both around the microimplant and within GMS channels. Apart from connective tissue, amorphous masses were also found in the proximal portion of the microimplant. It cannot be excluded that due to nutritional disorders there came to the degeneration of collagenized connective tissue.
The presence of giant cells both around and inside the microimplant may point to the role of activated monocytes and macrophages in the pathomechanism of the development of GMS obstruction. Distinct inflammatory infiltrations from mononuclear cells were also observed in the specimens. Their occurrence proves specific immune response to the antigen presented by macrophages.
Summing up, it should be concluded that microimplant obstruction caused by the connective tissue proliferation is the cause of the failure of surgery with GMS. This process can be non-inflammatory, but it can also result from chronic inflammation in the suprachoroidal space.

References
Emi K, Pederson JE, Toris CB . Hydrostatic pressure of the suprachoroidal space. Invest Ophthalmol Vis Sci 1989; 30: 233–238.
Melamed S, Ben Simon GJ, Goldenfeld M, Simon G . Efficacy and safety of gold micro shunt implantation to supraciliary space in patients with glaucoma: a pilot study. Arch Ophthalmol 2009; 127: 264–269.
Saheb H, Ahmed II . Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol 2012; 23: 96–104.
Figus M, Lazzeri S, Fogagnolo P, Iester M, Martinelli P, Nardi M . Supraciliary shunt in refractory glaucoma. Br J Ophthalmol 2011; 95: 1537–1541.
Mastropasqua L, Agnifili L, Ciancaglini M, Nubile M, Carpineto P, Fasanella V et al. In vivo analysis of conjunctiva in gold micro shunt implantation for glaucoma. Br J Ophthalmol 2010; 94: 1592–1596.
Agnifili L, Costagliola C, Figus M, Iezzi G, Piattelli A, Carpineto P et al. Histological findings of failed gold micro shunts in primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 2012; 250: 143–149.
Acknowledgements
We thank a professional translator who translated and corrected the English language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This paper was presented (oral presentation) during the 6th International Congress on Glaucoma Surgery, Glasgow, Scotland, 13–15 September 2012.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Rękas, M., Pawlik, B., Grala, B. et al. Clinical and morphological evaluation of gold micro shunt after unsuccessful surgical treatment of patients with primary open-angle glaucoma. Eye 27, 1214–1217 (2013). https://doi.org/10.1038/eye.2013.154
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2013.154
Keywords
This article is cited by
-
Conventional glaucoma implants and the new MIGS devices: a comprehensive review of current options and future directions
Eye (2021)
-
STARflo – ein suprachoroidales Drainageimplantat in der Glaukomchirurgie
Der Ophthalmologe (2018)
-
Safety and effectiveness of gold glaucoma micro shunt for reducing intraocular pressure in Japanese patients with open angle glaucoma
Japanese Journal of Ophthalmology (2017)