Abstract
Purpose
To elucidate changes in the neurosensory retina in the macular area, using spectral domain OCT and correlate with functional loss on fundus-related microperimetry, in patients with diabetes and no diabetic retinopathy compared with age-matched healthy volunteers.
Methods
This was a prospective study enroling 39 patients in each group. All patients underwent comprehensive dilated eye examination. The foveal thickness and the photoreceptor layer thickness at the foveal centre were measured using spectral domain OCT, and the mean retinal sensitivity of central 20 degrees was measured using microperimetry.
Results
The mean age of the patients with diabetes was 50.92±4.75 years, and of controls, 49.87±5.50 years. SD-OCT measured photoreceptor layer thickness (PLT) to be 61.62±4.48 μm in cases, and 68.79±7.84 μm in controls (P<0.0001); foveal thickness (FT) was 168.64±16.46 μm in cases and 177.74±14.58 μm in controls (P=0.012). The mean retinal sensitivity (MRS) of the central 20 degrees, measured on microperimetry was 15.74±3.74 db in cases and 17.70±1.5 db in controls (P<0.003). In cases compared with controls (aged under 50 years) statistically significant differences were noted in all the three outcome variables: FT, P=0.030; PLT, P=0.015; and MRS, P=0.020. The duration of diabetes influenced only the PLT (P=0.017). Statistical analysis was performed with Student's t-test and χ2 test.
Conclusion
Neuronal damage was observed in those eyes that did not have clinical evidence of diabetic retinopathy.
Similar content being viewed by others
Introduction
Diabetic retinopathy, one of the microvascular complications of diabetes mellitus, causes visual loss in the working age group.1 Despite advances in treatment such as laser photocoagulation, intravitreal injection of anti-VEGF (Vascular Endothelial Growth Factor) or vitreous surgery, vision does not improve significantly in many and in some, vision continues to decline.2, 3 The most commonly accepted pathophysiological model for this visual loss is attributed to vascular change (microvascular theory) in the retina, particularly in retinal capillaries: capillary dilatation and increased permeability leading to microaneurysms, haemorrhages, retinal exudates, endothelial cell proliferation and so on.4 The other pathophysiological model, somewhat less investigated, is the neurodegenerative theory.5, 6 The neurodegenerative changes are apoptosis of several neuronal cells including ganglion, amacrine, horizontal, Muller and photoreceptor cells; these changes probably precede microvascular changes.5 These changes explain some of the functional deficits in vision occurring early in the course of the disease. These changes have been studied with neurophysiological, psychometric, histopathological and biochemical experiments, and are supported by the electrophysiological and clinical evidence such as pattern electroretinogram, contrast sensitivity and colour vision.7, 8, 9
Optical coherence tomography (OCT) provides reliable, reproducible, and objective retinal images and permits quantitative assessment in diabetic macular oedema.10 Spectral domain OCT is a newer-generation high-resolution OCT with the advantages of high-speed data acquisition, three-dimensional reconstruction of acquired retinal images, and improved visualisation of retinal architecture, layer by layer.11, 12 Microperimetry (fundus-related perimetry) has been developed to quantify the functional loss by determining the retinal threshold in the macular area and correlating it with the precise location of oedema (or thickness by optical coherence tomography).13, 14
The purpose of this study is to identify the architectural derangement in neural retina in individuals with diabetes having no diabetic retinopathy and compare with healthy controls, using Spectral Domain Optical Coherence Tomography (SD-OCT) and then correlating these anatomical changes with functional loss on microperimetry. The study results might pave way for newer and novel therapeutic approaches—such as neuroprotective treatment—as an adjunct to available options.
Materials and methods
This was a prospective cross-sectional study involving 39 diabetic cases and 39 control healthy participants. The diabetic participants (cases) were recognised based on either the history of known diabetes mellitus, or if the subjects were on treatment for diabetes. All the patients underwent comprehensive eye examination including 45° digital fundus photographs; examination of the right eye alone was done in all the participants, for ease of comparison. We compared the changes observed on SD-OCT in terms of foveal thickness and photoreceptor layer thickness measured in microns and microperimetry in terms of mean retinal sensitivity of the central 20 degrees expressed in decibels in 39 participants with diabetes, but no diabetic retinopathy (cases) with 39 healthy individuals with no diabetes (controls). The study was approved by the Institutional Review Board.
Inclusion and exclusion criteria
Inclusion criteria included enroling individuals with or without diabetes, above the age of 40 years; an informed consent was signed by all of the study participants. Exclusion criteria included were best-corrected visual acuity of 20/200 or less; other ocular diseases such as cataract, glaucoma, diabetic retinopathy, age-related macular degeneration or any other macular disorders; refractive error of more than 6.0 diopter; poor fixation; pupillary size of less than 4 mm; significant media haze; and history of intraocular surgery or laser treatment.
SD-OCT
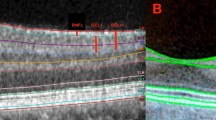
The foveal centre was defined by a hyper-reflective dot echo at the innermost retinal layer. All the measurements were carried out at the same point for the uniformity of comparison. The thickness of the foveal neurosensory retina was measured (in microns) by measuring the distance between the red line at the hyper-reflective echo of the innermost retinal layer (representing internal limiting membrane) and the sky blue line at the inner edge of the retinal pigment epithelium (Figure 1a). The photoreceptor layer thickness (PLT) at the fovea was measured (in microns) by measuring the distance between the inner edge of the retinal pigment epithelial layer (sky blue line) and the external limiting membrane. SD-OCT features (Copernicus, Optopol Technologies, Zawierci, Poland) included asterix scan (6 B-scans per second, 2742 A-scans per B-scan), and the length of each scan was 7 mm. All measurements were performed manually by a single observer.
(a) Spectral domain OCT photograph of the macula shows three landmarks: internal limiting membrane, external limiting membrane, and the retinal pigment epithelium. A hyper-reflective echo at the innermost retinal layer, in the inset, represents the foveal centre. (b) Fundus-related microperimetry record shows the retinal sensitivity values in decibels and in false colour coding.
Microperimetry
Using automated fundus-related perimeter, (MP1 Microperimeter, Nidek Technologies, Padova, Italy) the fundus was imaged in real time on a video monitor with an infra-red fundus camera (1392 × 1038 pixel resolution, 45° field). The infra-red camera, using auto-tracking followed the patient's eye movement and software compensated for stimulus projection location. The data was registered on a colour picture, and the retinal sensitivity was expressed in decibels. The specifications used in the study included central 20° area, 33 stimulation points, 4-2 threshold, duration of stimulus being 120 ms, Goldmann size III stimulus, white background with intensity of 4 apostilbs (Figure 1b).
Statistical analysis
Statistical analyses were performed using SPSS (Statistical Package for Social Sciences, version 14.0, Chicago, IL, USA). The results were expressed as mean±SD if the variables were continuous, and as percentage, if categorical. Student's t-test for comparing continuous variables, and χ2-test, to compare proportions among groups were used. The P-value of ⩽0.05 was considered significant. Spearman's coefficient was used in the analysis of correlation between retinal thickness and retinal sensitivity.
Results
The study comprised of 78 participants, 39 cases with diabetes mellitus but without diabetic retinopathy, and 39 controls with no diabetes mellitus. There were 43 (55.1%) men and 35 (44.8%) women. Cases consisted of 17 (43.6%) men and 22 (56.4%) women, whereas the controls consisted of 26 (66.6%) men and 13 (33.3%) women. The mean age was 50.92±4.75 years and 49.87±5.50 years, in cases and controls, respectively. Median Snellen's visual acuity was 20/20 in both the groups.
Table 1 summarises the difference in the outcome measures between cases and controls. SD-OCT measured PLT to be 61.62±4.48 μm in cases, and 68.79±7.84 μm in controls (P<0.0001); the FT was 168.64±16.46 μm and 177.74±14.58 μm, in cases and controls, respectively (P=0.012). Retinal sensitivity of the central 20°, measured on microperimetry, was 15.75±3.74 db in cases, and 17.7±1.5 db, in controls (P<0.003).
Figure 2 shows the microperimetry and SD-OCT records of a control and three cases. As the thickness of the foveal area and photoreceptor layer, as measured on SD-OCT, reduces, the retinal sensitivity, as measured on microperimetry and shown on colour coding, also becomes less.
Control, shows normal macular architecture and normal retinal sensitivity (represented by green colour). Case 1 to case 3 shows gradual decline in foveal and photoreceptor thickness and corresponding increase in yellow or red colour on microperimetry (representing reduced sensitivity). (a and b) Microperimetry and SD-OCT pictures of a control patient showing normal retinal sensitivity and central foveal and photoreceptor layer thickness. (c and d) Microperimetry, and SD-OCT pictures of a patient with mild neuronal damage. (e and f) Microperimetry and SD-OCT pictures of a patient with moderate neuronal damage. (g and h) Microperimetry and SD-OCT pictures of a patient with severe neuronal damage showing markedly reduced retinal sensitivity and correspondingly reduced retinal thickness.
Table 2 shows the influence of age and gender on the three outcome variables. Age group less than 50 years showed a statistically significant difference in the outcome measures in cases as compared with controls (FT, P=0.030; PLT, P=0.015; MRS, P=0.020). PLT showed a highly statistically significant decrease in cases as compared with controls in the age group ⩾50 years. Both gender showed a statistically significant decrease in the mean PLT in cases compared with controls; however, significant difference with respect to MRS was observed only in men.
Table 3 shows the influence of duration of diabetes and glycosylated haemoglobin on outcome variables in cases. Increased duration of diabetes was inversely related to PLT (P=0.017) only, and not the FT and retinal sensitivity. Glycosylated Hb did not affect the outcome variables.
Discussion
The correlation of SD-OCT and microperimetry parameters for the experimental evidence of early neuronal damage in diabetic participants without clinical evidence of diabetic retinopathy has not been explored in the past. The present age-matched case–control study showed that in such individuals with diabetes but no clinical diabetic retinopathy, all of the three outcome variables namely mean FT, mean PLT and MRS were reduced in comparison with controls. The results provided evidence that neural damage possibly precedes clinical diabetic retinopathy. Previous studies have noted mixed results when comparing the macular thickness in type II diabetic participants with no retinopathy and control non-diabetic participants.15, 16, 17 The measurements were recorded with the Stratus OCT, with some showing increased macular thickness in diabetic patients,15, 16 whereas others showing no such correlation.17
Recent evidence suggests that the selective thinning of inner retinal layers supports the concept of early neurodegenerative component in patients with minimal diabetic retinopathy.18 Sufficient evidence also comes from the animal studies that thickness of the inner retinal layers decreases in diabetic condition, indicating early neurodegeneration.19, 20
Although apoptosis in the retinal capillary pericytes is known to cause the early microvascular changes seen in diabetic retinopathy,21 gradual loss of neurons resulting from ganglion cell atrophy and degeneration of the inner nuclear and inner plexiform layers in diabetics has been accepted as an evidence for the early neurodegeneration occurring in diabetic retinopathy.22 What remains to be proven is the sequence of events, that is, whether the apoptotic neuronal cell changes precede the vascular damage, or vice versa.
Although there are reports evaluating central macular function in subjects with diabetic macular oedema using microperimetry with scanning laser ophthalmoscope,23 fundus-related microperimeter24 and multifocal ERG,25 correlation of decreased retinal sensitivity as a corollary to reduced retinal thickness (in participants with no clinical diabetic retinopathy or maculopathy), as measured on spectral domain OCT and MP has not been reported earlier.
This study, in a sub-group analysis, showed significant decrease in all of the three outcome variables in participants with less than 50 years of age, in cases than controls. For participants with more than 50 years of age, only PLT was affected. Increasing age has been noted to have inverse relation with macular thickness in some studies,26 whereas others have not observed this relationship.27, 28
Although poor glycemic control is known to worsen diabetic macular oedema,17, 29 its influence on retinal thickness in patients with no diabetic retinopathy has not been elucidated in this report. However, increased duration of diabetes did reduce PLT. Clinically as well, as the duration of hyperglycaemia increases, the risk of developing diabetic retinopathy or maculopathy rises.30
Several limitations exist in this study. Absence of clinical diabetic retinopathy was based on ophthalmic examination alone. The techniques such as vitreous fluorometry or fundus fluorescein angiogram, used for the early detection of subclinical diabetic retinopathy, would have helped define the study population better.31, 32, 33 Further longitudinal studies with proper selection of the cohort and using additional functional tests such as multifocal ERG would be required in the future to confirm the present hypothesis.
We compared the changes in the central foveal region in the cases and controls; however, the inner retinal layers are absent in the foveal region. Hence, these changes cannot be generalised for the parafoveal thickness measurements. Further studies would be required for the globalisation of the early neurodegenerative changes in diabetic retina.
As newer approach to the patho-physiology of diabetic retinopathy is being investigated by the researchers, the question as to whether the microvascular damage or the neurodegenerative damage is the initiating event is becoming the focus of interest. The results of this paper add to the growing evidence about the alternate pathogenic mechanisms in diabetic retinopathy, that is, neurodegenerative damage. However, the ability of retinal physiological tests soon after the diagnosis of diabetes mellitus to identify patients with a high risk of developing diabetic retinopathy would need to be confirmed in large, longitudinal studies. Once it is confirmed, there would be a paradigm shift in the philosophy, the way we manage diabetic retinopathy today. Future treatment agents would focus on recovery of neural damage besides treating vascular damage and thereby improving the chances of better visual outcomes.
Conflict of interest
The authors declare no conflct of interest.
References
Klein R, Klein BEK . Vision disorders in diabetes. In: National Diabetes Data Group (eds). Diabetes in America, 2nd ed. NIH (publ. no. 95-1468): Bethesda, MD, 1995, pp 293–338.
Blankenship GW . Fifteen-year argon laser and xenon photocoagulation results of Bascom Palmer Eye Institute's patients participating in the diabetic retinopathy study. Ophthalmology 1991; 98: 125–128.
Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes 1997; 46: 1473–1480.
Cunha-Vaz J, Faria de Abreu JR, Campos AJ . Early breakdown of the blood-retinal barrier in diabetes. Br J Ophthalmol 1975; 59: 649–656.
Barber AJ . A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27: 283–290.
Lieth E, Gardner TW, Barber AJ, Antonetti DA . Retinal neurodegeneration: early pathology in diabetes. Clin Exp Ophthalmology 2000; 28: 3–8.
Falsini B, Porciatti V, Scalia G, Caputo S, Minnella A, Di Leo MA et al. Steady-state pattern electroretinogram in insulin-dependent diabetics with no or minimal retinopathy. Doc Ophthalmol 1989; 73: 193–200.
Sokol S, Moskowitz A, Skarf B, Evans R, Molitch M, Senior B . Contrast sensitivity in diabetics with and without background retinopathy. Arch Ophthalmol 1985; 103: 51–54.
Greenstein V, Sarter B, Hood D, Noble K, Carr R . Hue discrimination and S cone pathway sensitivity in early diabetic retinopathy. Invest Ophthalmol Vis Sci 1990; 31: 1008–1014.
Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT . Evaluation of time domain and spectral domain optical coherence tomography in the measurement of diabetic macular edema. Invest Ophthalmol Vis Sci 2008; 49: 4290–4296.
Wojtkowski M, Bajraszewski T, Targowski P, Kowalczyk A . Realtime in vivo imaging by high-speed spectral optical coherence tomography. Opt Lett 2003; 28: 1745–1747.
Schmidt-Erfurth U, Leitgeb RA, Michels S, Povazay B, Sacu S, Hermann B et al. Three-dimensional ultrahigh-resolution optical coherence tomography of macular diseases. Invest Ophthalmol Vis Sci 2005; 46: 3393–3402.
Midena E . Microperimetry. Arch Soc Esp Oftalmol 2006; 81: 183–186.
Vujosevic S, Midena E, Pilotto E, Radin PP, Chiesa L, Cavarzeran F . Diabetic macular edema: correlation between microperimetry and optical coherence tomography findings. Invest Ophthalmol Vis Sci 2006; 47: 3044–3051.
Lattanzio R, Brancato R, Pierro L, Bandello F, Iaccher B, Fiore T et al. Macular thickness measurements by optical coherence tomography (OCT) in diabetic patients. Eur J Ophthalmol 2002; 12: 482–487.
Sanchez-Tocino H, Alvarez-Vidal A, Maldonado MJ, Moreno-Montanes J, Garcia-Layana A . Retinal thickness study with optical coherence tomography in patients with diabetes. Invest Ophthalmol Vis Sci 2002; 43: 1588–1594.
Asefzadeh B, Fisch BM, Parenteau CE, Cavallerano AA . Macular thickness and systemic markers for diabetes in individuals with no or mild diabetic retinopathy. Clin Exp Ophthalmol 2008; 36: 455–463.
Van Dijk HW, Kok PH, Garvin M, Sonka M, De Vries JH, Michels RP et al. Selective loss of inner retinal layer thickness in type I diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci 2009; 50: 3404–3409.
Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB . Death of retinal neurons in streptozocin-induced diabetic mice. Invest Ophthalmol Vis Sci 2004; 45: 3330–3336.
Barbar AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci 2005; 46: 2210–2218.
Li W, Yanoff M, Liu X, Ye X . Retinal capillary pericyte apoptosis in early human diabetic retyinopathy. Chin Med J (Engl) 1997; 110: 659–663.
Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW . Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 1998; 102: 783–791.
Mori F, Ishiko S, Kitaya N, Hikichi T, Sato E, Takamiya A et al. Use of scanning laser ophthalmoscope microperimetry in clinically significant macular edema in type 2 diabetes mellitus. Jpn J Ophthalmol 2002; 46: 650–655.
Okada K, Yamamoto S, Mizunoya S, Hoshino A, Arai M, Takatsuna Y . Correlation of retinal sensitivity measured with fundus-related microperimetry to visual acuity and retinal thickness in eyes with diabetic macular edema. Eye 2006; 20: 805–809.
Yamamoto S, Yamamoto T, Hayashi M, Takeuchi S . Morphological and functional analysis of diabetic macular edema by optical coherence tomography and multifocal electroretinograms. Graefe's Arch Clin Exp Ophthalmol 2001; 239: 96–101.
Eriksson U, Alm A . Macular thickness decreases with age in normal eyes. A study on the macular thickness map protocol in the Stratus OCT. Br J Ophthalmol 2008; e-pub ahead of print, 19 November 2008; doi:10.1136/bjo.2007.131094.
Chan A, Duker JS, Ko TH, Fujimoto JG, Shuman JS . Normal macular thickness measurements in healthy eyes using Stratus optical coherence tomography. Arch Ophthalmol 2006; 124: 193–198.
Massin P, Erginay A, Haouchine B, Mehidi AB, Paques M, Gaudric A . Retinal thickness in healthy and diabetic subjects measured using optical coherence tomography mapping software. Eur J Ophthalmol 2002; 12: 102–108.
Chou TH, Wu PC, Kuo JZC, Lai CH, Kuo CN . Relationship of diabetic macular oedema with glycosylated haemoglobin. Eye 2008; 1–4.
Klein R, Klein BE, Moss SE, Cruickshanks KJ . The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology 1995; 102: 7–16.
Cunha Vaz JG, Fonseca JR, Abreu JF, Ruas MA . Detection of early retinal changes in diabetes by vitreous fluorophotometry. Diabetes 1979; 28: 16–19.
Rains MF . Vitreous fluorophotometry. A review. J R Soc Med 1988; 81: 403–406.
Gao LQ, Zhang F, Zhou HY, Yan W, Xiong Y, Wang GL . Comparison of fundus photography and fluorescein angiography in grading diabetic retinopathy. Zhonghua Yan Ke Za Zhi 2008; 44: 12–16.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in parts at the Asia-ARVO 2009, Hyderabad, India (January 15–18, 2009)
Rights and permissions
About this article
Cite this article
Verma, A., Rani, P., Raman, R. et al. Is neuronal dysfunction an early sign of diabetic retinopathy? Microperimetry and Spectral Domain Optical Coherence Tomography (SD-OCT) Study in individuals with diabetes, but no diabetic retinopathy. Eye 23, 1824–1830 (2009). https://doi.org/10.1038/eye.2009.184
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2009.184
Keywords
This article is cited by
-
Contrast sensitivity function under three light conditions in patients with type 1 diabetes mellitus without retinopathy: a cross-sectional, case–control study
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Multifocal pupillographic objective perimetry for assessment of early diabetic retinopathy and generalised diabetes-related tissue injury in persons with type 1 diabetes
BMC Ophthalmology (2022)
-
Association of inner retinal reflectivity with qualitative and quantitative changes in retinal layers over time in diabetic eyes without retinopathy
Eye (2022)
-
Changes in retinal layers in type 1 diabetes mellitus without retinopathy measured by spectral domain and swept source OCTs
Scientific Reports (2021)
-
Structural and functional findings in patients with moderate diabetic retinopathy
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)