Abstract
Purpose
To evaluate the rotational stability of the Acrysof Toric intraocular lens (IOL) after experimental eye trauma.
Methods
Two human cadaver eyes were prepared in accordance with the Miyake–Apple technique, with closed-system modification. After phacoemulsification, an Acrysof Toric IOL was implanted in the capsular bag. Experimental traumatisation of the globe was achieved by pressing against the eye wall using a cotton tip or a fingertip, whereas the IOL was observed from the posterior view. Digital photographs of the eye before and after the procedures were overlaid to detect and measure rotation of the IOL.
Results
The IOL rotated 5.80° when the traumatic procedures did not cause important leakage from the incision. When the traumatic procedures caused important leakage from the incision and anterior chamber collapse, the IOL rotated 41.00°.
Conclusion
Ocular trauma can cause rotation of the Acrysof Toric IOL. In the event of an eye trauma with no or insignificant leakage from the incision, the IOL rotates less than when the trauma causes significant leakage from the incision.
Similar content being viewed by others
Introduction
Pre-existing corneal astigmatism is an important limiting factor for optimal results of cataract surgery in a significant number of patients. Treatment of astigmatism with toric IOL implantation has theoretic advantages over that of keratorefractive procedures, as it does not require additional corneal manipulation. In addition, it requires no special skills and expensive instrumentation other than accurate surgical placement of the IOL aligned with the corneal cylinder axis. The major concern is the possibility of rotation of the IOL in the capsular bag after implantation. The purpose of this study is to evaluate the rotational stability of the Acrysof Toric IOL after experimental eye trauma, in a Miyake–Apple preparation.
Materials and methods
Two human cadaver eyes unsuitable for therapeutic purposes were prepared according to the Miyake–Apple technique,1 with the closed-system modification.2 After phacoemulsification and aspiration through a 2.75 mm clear corneal incision, an Acrysof Toric IOL model SN60T4 (2.25 D cylinder power, Alcon, Fort Worth, Texas, USA) was implanted in the capsular bag. Subsequently, the globe was experimentally traumatised by repeatedly poking the eye wall with a cotton tip applicator or a fingertip (Figure 1). The strokes were of variable strength, randomly applied on the sclera near the limbus and on the cornea throughout the globe circumference, and repeated in short intervals (approximately 2 to 5 strokes per second). The behaviour of the IOL in the capsular bag was observed from the posterior view when the eye was manipulated. The first eye was implanted with a 17.5 D IOL. In the second eye, the incision was enlarged to 5.0 mm. It was first implanted with a 6.5 D IOL, subsequently exchanged for a 30.0 D IOL following the traumatic procedures.
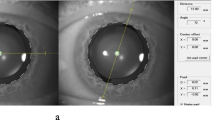
Digital photographs taken from the digital video recordings were analysed with an image editing software (LiveQuartz, Paris, France; version 1.8.1). Images of the eye from the Miyake–Apple posterior view before and after the trauma were overlaid, aligning the IOL optics. The three peripheral dots that mark the cylindrical axis on each half of the IOL optics were used as references to measure the rotation of the lens directly on the computer screen using the Screen Protractor software version 3.2 (Iconico Inc., New York, NY, USA).
We certify that all applicable institutional and governmental regulations concerning the ethical use of human tissue were followed during this research.
Results
In the first eye, the IOL rotated 5.80° (counterclockwise from the posterior view, clockwise from the anterior view or a surgeon's perspective) after traumatisation of the eye (Figure 2). During the manoeuvres, there was no sudden or bulky aqueous leakage through the 2.75 mm clear corneal incision. The traumatic procedures were applied to the eye for 12 min long. In the second eye, the trauma caused important leakage from the 5.0 mm incision, causing anterior chamber collapse and hypotony. In this eye, we observed rotation of both the 6.5 D IOL and of the 30.0 D IOL. The 6.5 D IOL rotated 41.00° counterclockwise (posterior view) (Figure 3). The 30.0 D IOL, implanted after the explantation of the 6.5 D IOL, similarly rotated 41.00° counterclockwise (Figure 4). The traumatic procedures were carried out on these eyes for 5 min.
Discussion
Intraocular lens dislocation is a known complication after eye trauma. However, to the best of our knowledge, rotation of an implanted IOL after ocular trauma has not yet been reported in the literature. Rotation of an IOL can occur up to 3 months after implantation, but is more likely to happen during the first 2 weeks postoperatively, before capsular fusion traps the lens in the bag.3
Different methods have been used to verify IOL rotation. In a clinical setting, when examining a patient and acquiring images in different periods of time, head position, direction of gaze, cyclorotation of the eye, parallaxes and position of the camera are important factors that may cause errors of interpretation.4 The Miyake–Apple preparation in this study offers advantages over clinical studies because the images are taken on the same setting and it allows real-time visualisation of the IOL during trauma. As the eye is fixed to the glass slide and to the table with the microscope and the camera, errors from misalignment of the eye and the camera are eliminated. This study is the first to use the image editing and on screen protractor softwares described in the Materials and methods section. We found this method easy to use and reliable.
Our observations suggest that if force is applied to an eye and is insufficient to cause significant leakage from the incision, minor degrees of rotation of the IOL can be expected. If the force is sufficient to cause significant leakage from the incision, considerable degrees of rotation can be expected. The rotation of 5.80° observed in the first experiment would decrease the IOL cylindrical correction by approximately 19%. This rotation took 12 min of repeated traumatisation to the eye to occur, and was not noticed by the surgeon during the experiment when observing on the monitor. In the other experiments, the surgeon stopped the manoeuvres after 5 min, because rotation of the IOL had already been noticed. The rotation of 41.00° of the IOL would have added approximately 35% of the IOL cylinder power to a patient's preoperative astigmatism.
Our study has some limitations. First, owing to limited availability of human cadaver eyes, we did not have more eyes to repeat the experiment on a larger sample and reproduce our findings. Second, the Miyake–Apple preparation may not be a good model for eye trauma in a real-life situation. Although it offers already stated advantages for this study, the need to section the eye and fix it to a glass slide limits the spectrum of traumatic procedures that can be tested, and probably changes the eye responses to these procedures. Third, because of the lack of standardisation and measurement of the traumatic stimuli, we could not correlate specific location or strength of the trauma to IOL rotation. The repetitive traumatic stimuli applied may not simulate a real-life occurring trauma, but may simulate intense repetitive eye rubbing. Further studies with a larger number of eyes and with standardised traumatising procedures in a more controlled method or with different experimental models are warranted.
With the increasing number of toric IOL implantations, both surgeons and patients should be aware of the possibility of rotation of the toric IOL in the event of an ocular trauma in the postoperative period. Patients receiving a toric IOL implantation should be advised not to rub their eyes and to take extra protection against ocular trauma during the early postoperative period. More resistant incisions would also prevent IOL rotation in the occurrence of a postoperative trauma. This could be achieved by making the smallest possible incision that allows the implantation of the IOL, which would at the same time be advantageous on minimising surgically induced astigmatism, or by suturing the incision, which on the other hand could induce unpredictable astigmatism.
References
Davis BL, Nilson CD, Mamalis N . Revised Miyake-Apple technique for postmortem eye preparation. J Cataract Refract Surg 2004; 30 (3): 546–549.
Auffarth GU, Wesendahl TA, Solomon KD, Brown SJ, Apple DJ . A modified preparation technique for closed-system ocular surgery of human eyes obtained postmortem: an improved research and teaching tool. Ophthalmology 1996; 103 (6): 977–982.
Patel CK, Ormonde S, Rosen PH, Bron AJ . Postoperative intraocular lens rotation: a randomized comparison of plate and loop haptic implants. Ophthalmology 1999; 106 (11): 2190–2195.
Viestenz A, Seitz B, Langenbucher A . Evaluating the eye′s rotational stability during standard photography: effect on determining the axial orientation of toric intraocular lenses. J Cataract Refract Surg 2005; 31 (3): 557–561.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sources of public and private financial support: Intraocular lenses provided by Alcon Laboratories, Sydney, Australia.
Financial interest: None.
This work was presented as a film on the Australasian Society of Cataract and Refractive Surgeons (AUSCRS) Annual Meeting, Coolum, Australia, June 2008, and on the 40th Annual Scientific Congress of the Royal Australian and New Zealand College of Ophthalmologists (RANZCO), Melbourne, Australia, November 2008.
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pereira, F., Milverton, E. & Coroneo, M. Miyake–Apple study of the rotational stability of the Acrysof toric intraocular lens after experimental eye trauma. Eye 24, 376–378 (2010). https://doi.org/10.1038/eye.2009.150
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2009.150