Abstract
Purpose
To compare the thickness of the ciliary bodies of eyes with unilateral high axial myopia with their relatively normal fellow eyes.
Methods
A total of 19 patients with unilateral high axial length (AL) were included in the study. Mean patient age was 28.4±10.4 (11–44) years. All eyes underwent ultrasound biometry to measure the AL, and ultrasound biomicroscopy to measure the anterior chamber depth, ciliary body thickness (CBT), and ciliary process thickness (CPT), ciliary muscle thickness (CMT). The results were compared between each subject's high myopic eye and relatively normal fellow eye.
Results
The mean AL was 27.24±1.52 mm (range: 25.16–30.21 mm) in high myopic eyes and 23.64±0.86 mm (range: 22.47–25.10 mm) in normal fellow eyes. The median ±95% confidence interval of CBT, CPT, and CMT was 1.350±0.034, 0.626±0.072, and 0.698±0.057 mm, respectively, in high myopic eyes and 1.211±0.050, 0.535±0.064, and 0.644±0.065 mm, respectively, in normal fellow eyes. The anterior chamber depth, CBT, CPT, and CMT were significantly higher in myopic eyes compared with their relatively normal fellow eyes (P<0.05). CMT significantly increased with age in both groups (P<0.05). There was no significant correlation between age and CBT in both the groups (P>0.05).
Conclusion
The CBT, CMT, and CPT are significantly higher in eyes with unilateral high axial myopia than in their relatively normal fellow eyes.
Similar content being viewed by others
Introduction
The main structural difference between hyperopic and myopic eyes is the axial length (AL), which is higher for myopic eyes.1, 2, 3 Using magnetic resonance, it has been documented that myopic eyes also have larger axes in the other two dimensions (equatorial and vertical axes) as well.4, 5 Equatorial enlargement during ocular growth may increase tension on the zonules and may affect ciliary body thickness (CBT).6, 7
Ultrasound biomicroscopy (UBM) allows in vivo, real-time imaging of the ciliary region, including structures not otherwise visible, and provides a digital image from which morphometric measurements can be readily made.8, 9, 10, 11, 12
Although Oliveira et al13 reported a positive correlation between AL and CBT, their measurements may be influenced by individual variability and age.13, 14, 15 To minimize these influences in this study, we compared the CBT of eyes with unilateral high axial myopia with same subject's fellow eyes with relatively normal AL, using UBM.
Methods
Subjects with unilateral high myopia were enrolled from the patients who visited Ankara University School of Medicine between November 2004 and September 2006. A total of 19 subjects (7 men, 12 women) with unilateral high myopia who have cycloplegic spherical equivalent (c-SE) more than −6.00 D in one eye and equal or more than −5.00 D difference between each eye were included in the study. Exclusion criteria were presence of central nerve system or systemic disease or syndrome, presence or history of ocular disease other than high myopia, previous ocular surgery or trauma, and use of systemic or ocular medication. This study followed the tenets of the Declaration of Helsinki and was approved by the institutional review board. Informed consent was obtained from each subject after explanation of the nature of the study. No subjects were excluded other than the exclusion criteria.
Subjects who were enrolled underwent an extensive ophthalmologic examination that included visual acuity measurements, manifest and cycloplegic refraction, slit-lamp biomicroscopy, Goldmann tonometry and fundus examination. The eyes with high myopia were defined as the ‘study eyes’ and the fellow eyes of the same subjects were defined as the ‘control eyes’.
At 40 min after instillation of three drops of cyclopentolate 1% (5 min between each drop), all eyes underwent AL measurements with ultrasound biometry (Occuscan, Alcon Inc., Fort Worth, TX, USA) and UBM (Model P40; Paradigm Medical Industries, Salt Lake City, UT, USA) to evaluate the ciliary body. A single well-trained observer, who was masked about the results of AL and other measurements, performed all the UBM studies. UBM was performed at the temporal corneoscleral limbus with a 50 MHz transducer probe. After surface anaesthesia was achieved with proparacaine 0.5%, an eyecup filled with 2% methylcellulose was applied to the eyeball between the eyelids. The subject was asked to fixate on the distance target at the ceiling with the fellow eye. Examination was performed under room light. Fine movements of the UBM probe were required to explore the areas of interest, always perpendicular to the surface of the globe.
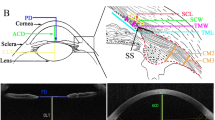
Measurements were performed using the built-in caliper. All of the measurements were made from three different UBM images and the mean of these three measurements was used for the analysis. An image through the centre of the pupil was recorded for anterior chamber depth (ACD) measurement (measured from the corneal endothelium to the anterior lens surface). An ultrasound biomicroscope scan of a parallel (to limbus) section taken at the thickest part of the ciliary body was used for the CBT measurements. The CBT was measured from the tip of the ciliary process to the sclera, the thickness of the ciliary muscle (CMT) was measured from the base of the ciliary process to the sclera, and the thickness of ciliary process (CPT) was measured from the tip to the base of the ciliary process (Figures 1 and 2). The mean of three ciliary processes (the longest ones) in the image was used for the CPT measurements.
The mean difference in each parameter (d-parameter) was calculated as: d-parameter=parameter (high myopic eye)−parameter (fellow eye).
All statistical data were analysed using the SPSS 11.0 (SPSS Inc., USA) statistical software. Normality of data in each group was tested by evaluating the normal probability plots and Shapiro–Wilk test. As the distributions of the data were not normal, correlations were tested using the Spearman's rank correlation. Statistical differences were considered significant when P<0.05.
Results
The mean age of the subjects was 28.4±10.4 (median ±95% confidence intervals (CIs): 27±5.0, range: 11–44) years. The median ±95% CI best-corrected visual acuity, c-SE, AL, ACD, CBT, CMT, and CPT of high myopic and relatively normal fellow eyes, and the differences are demonstrated in Table 1. The best-corrected visual acuity, c-SE, AL, ACD, CBT, CMT, and CPT were significantly higher in high myopic eyes than those of their fellow eyes (P<0.001).
The distribution of magnitude of d-CBT, d-CPT, and d-CMT are given in Table 2. There was no significant correlation between d-cSE, d-AL, age and d-ACD, d-CBT, and d-CMT (P>0.05). Also, there was no significant correlation between AL, SE and CBT, CMT, and CPT in all eyes (P>0.05, combining study and control groups) except significant positive correlation between AL and CBT (r=0.38, P=0.020) and CMT (r=0.41, P=0.020).
Correlations between age and c-SE, AL, ACD, CBT, CMT, and CPT within each group are demonstrated in Table 3. There was just a statistically significant increase (P=0.051) in CPT in the control group with age and a significant decrease in CMT with age in both groups.
The mean standard deviation of the measurements of CBT, CPT, and CMT from three different UBM scan samples for 38 eyes to evaluate variability were 0.024±0.019 (range: 0.009–0.055), 0.025±0.020 (range: 0.006–0.059), and 0.012±0.011 mm (range: 0.001–0.045 mm), respectively.
Discussion
Assessment of ciliary body may be important for evaluation of glaucoma, zonules, lens, accommodation, and ciliary body's response to scleral surgery such as scleral expansion surgery for presbyopia. 16, 17, 18 Recently, Oliveira et al13 found a significant positive correlation between AL and CBT in 75 eyes with UBM. However, their results may be influenced by some factors: only 22 of 75 eyes in the study group of Oliveira et al were normal. Others had different types of glaucoma, including 14 (19%) eyes with narrow angle. It has been shown that ciliary bodies of eyes with narrow angle are thinner than those of normal control eyes.10 In addition, the mean age (51.8±16.5 years) in Oliveira et al's study was relatively high.13 Previous studies of the ciliary body, including the histologic studies, have documented a significant reduction of the area of the ciliary muscle due to atrophy,14, 15 and a significant change in configuration of the ciliary muscle with age.19, 20, 21 Furthermore, CBT measurements on a perpendicular (to limbus) UBM image with a constant distance (ie 1 mm) from the limbus may be affected by the change in configuration of the ciliary body with age, accommodation, and AL. Moreover, there might be individual (inherent) variability between the CBTs of subjects.
To overcome the possible influence of individual variability and age on the CBT measurements in our study, we measured CBTs of subjects with unilateral high axial myopia and compared the results with their fellow eyes with relatively normal AL. In addition, to minimize ciliary body configuration changes during accommodation,11, 22 we performed our UBM scanning 45 min after instillation of three drops of cyclopentolate 1%. Moreover, our study group was composed of predominantly young ages (mean age: 29.7±10.3 years) who were not significantly in the presbyopic age.
Our study showed that the CBT, CMT, and CPT are significantly higher in eyes with high axial myopia compared to those of relatively normal fellow eyes, independent of age. We also found significant positive correlation between AL and CMT supporting the findings of Oliveira et al.13 However, the magnitude of differences in CBT, CPT, and CMT between high myopic and relatively normal eyes varied and even there was no or little difference in some subjects. In addition, we could not find significant correlation between the difference in SE and AL vs difference in CBT, CPT, and CMT. These results may suggest that although ciliary body, ciliary processes, and ciliary muscle tend to be thicker in eyes with high axial myopia, AL does not seem to be the only determinant for the CBT, CPT, and CMT.
Currently, we do not know why the eyes with higher AL have higher CBT, CPT, and CMT. It was reported that choroid thickens as a response to deprivation myopia in some animals, which pushes the retina forward toward the image plane and causes the image plane back to the retina.23, 24 The same occurs if one puts a positive lens over the eye in chicks and monkeys.25, 26 The range of lens powers compensated for is greater in chicks than in monkeys, although monkeys can also compensate for stronger lenses if the lens power is stepped up gradually.25, 27 Similar mechanism may also be active in humans, however this entails further research.
It was demonstrated microscopically that ciliary muscle elastic tendons make extensive connections with the elastic layer of Bruch's membrane, which can transmit tension from the crystalline lens to the choroid and sclera by zonules and ciliary muscle.28 In myopes, the crystalline lens responds to ocular growth in the equatorial plane by thinning before the age of about 10 years. However, this response slows down after about 10 years of age probably due to increased lenticular stiffness.5, 7 Consequently, it was suggested that the zonules and ciliary body are exposed to increased tension due to the discrepancy between the crystalline lens and the growing eye.5, 7 It is possible that increased zonular tension from lenticular resistance may lead to an increased choroidal tension and thickening in ciliary body compensating the equatorial ocular growth.5, 6, 29
Previous studies showed that there is significant atrophy of the ciliary muscle with age.14, 15 However, we observed a significant increase in CMT with age in our study. This could be due to changes in configuration of the ciliary body of older unaccommodated eyes similar to the young accommodated state, namely anterior and inward displacement of the ciliary body with age.19, 20, 21 Although not significant, we observed a decrease in CPT with age in our study. However, there was no correlation between age and CBT, probably due to the increase in CMT and decrease in CPT with age.
Numerous prior studies have undertaken quantitative morphometric measurements using UBM.30, 31, 32, 33 Intraobserver reproducibility was reported to be high for various UBM measurements.12 Also, in this study ciliary body measurements using UBM seemed to be repeatable. However, the mean SD for the repeated UBM scans was highest for CPT measurements, followed by CBT and CMT measurements. This suggests that most reliable measurement in our study was for CMT, followed by CBT and CPT. The ciliary region measured from UBM images (the region between the inner surface of the sclera and the inner surface of the ciliary body) includes the ciliary muscle and perhaps the ciliary body ground plate and stroma.12, 14 However, it is impossible to distinguish between tissue types (such as muscle and connective tissue) with the UBM. Therefore, our CMT measurements probably include the thickness of both the muscle and the connective tissue.
Unilateral high axial myopia is not common.34 Weiss35 suggested that a high number children with unilateral high myopia have accompanying optic nerve or central nerve system abnormalities, whereas others36, 37, 38, 39, 40 did not report such a high incidence. It is possible that the results of Weiss may be biased because of being a tertiary centre for paediatric ophthalmology. Nevertheless, subjects who have accompanying abnormality other than typical findings of high myopia were not included in our study.
There were some limitations of our study. As unilateral high axial myopia is a rare condition, the number of subjects included in our study was limited. Further research, particularly longitudinal, with larger numbers of subjects with a wider range of age might show CBT, CMT, and CPT changes depending on age. It should also be taken into account that unilateral high myopes inherently may have different ciliary body configurations than bilateral high myopes.
In conclusion, our study showed that the thicknesses of the ciliary body, muscle, and processes are significantly higher in eyes with longer AL. Further studies are needed to investigate the clinical importance of this finding.
References
Carney L, Mainstone J, Henderson B . Corneal topography and myopia: a cross-sectional study. Invest Ophthalmol Vis Sci 1997; 38: 311–320.
Grosvenor T, Scott R . Role of the axial length/corneal radius ratio in determining the refractive state of the eye. Optom and Vis Sci 1994; 71: 573–579.
Llorente L, Barbero S, Cano D, Dorronsoro C, Marcos S . Myopic versus hyperopic eyes: axial length, corneal shape and optical aberrations. J Vis 2004; 4: 288–298.
Cheng HM, Singh OS, Kwong KK, Xiong J, Woods BT, Brady TJ . Shape of the myopic eye as seen with high-resolution magnetic resonance imaging. Optom and Vis Sci 1992; 69: 670–698.
Atchison DA, Jones CE, Schmid KL, Pritchard N, Pope JM, Strugnell WE et al. Eye shape in emmetropia and myopia. Invest Ophthalmol Vis Sci 2004; 45: 3380–3386.
Mutti DO, Zadnik K, Fusaro RE, Friedman NE, Sholtz RI, Adams AJ . Optical and structural development of the crystalline lens in childhood. Invest Ophthalmol Vis Sci 1998; 39: 120–133.
Zadnik K, Mutti DO, Fusaro RE, Adams AJ . Longitudinal evidence of crystalline lens thinning in children. Invest Ophthalmol Vis Sci 1995; 36: 1581–1587.
Pavlin CJ, Foster FS . Ultrasound Biomicroscopy of the Eye, 1st edn. Springer-Verlag: New York, 1995, pp 3–16.
Tello C, Liebmann J, Potash SD, Cohen H, Ritch R . Measurement of ultrasound biomicroscopy images: intraobserver and interobserver reliability. Invest Ophthalmol Vis Sci 1994; 35: 3549–3552.
Gohdo T, Tsumura T, Iijima H, Kashiwagi K, Tsukahara S . Ultrasound biomicroscopic study of ciliary body thickness in eyes with narrow angles. Am J Ophthalmol 2000; 129: 342–346.
Mishima HK, Shoge K, Takamatsu M, Kiuchi Y, Tanaka J . Ultrasound biomicroscopic study of ciliary body thickness after topical application of pharmacologic agents. Am J Ophthalmol 1996; 121: 319–321.
Bacskulin A, Gast R, Bergmann U, Guthoff R . Ultrasound biomicroscopy imaging of accommodative configuration changes in the presbyopic ciliary body. Ophthalmologe 1996; 93: 199–203.
Oliveira C, Tello C, Liebmann JM, Ritch R . Ciliary body thickness increases with increasing axial myopia. Am J Ophthalmol 2005; 140: 324–325.
Tamm S, Tamm E, Rohen JW . Age-related changes of the human ciliary muscle: a quantitative morphometric study. Mech Ageing Dev 1992; 62: 209–221.
Lutjen-Drecoll E, Tamm E, Kaufman PL . Age-related loss of morphologic responses to pilocarpine in rhesus monkey ciliary muscle. Arch Ophthalmol 1988; 106: 1591–1598.
Weinreb RN, Toris CB, Gabelt BT, Lindsey JD, Kaufman PL . Effects of prostoglandins on the aqueous humor outflow pathways. Surv Ophthalmol 2002; 47: 53–64.
Coca-Prados M, Edcribano J . New perspectives in aqueous humor secretion and in glaucoma: the ciliary body as a multifunctional neuroendocrine gland. Prog Retin Eye Res 2007; 26: 239–262.
Glasser A, Kaufman PL . Accommodation and presbyopia. In: Kaufman PL, Alm A (eds). Adler's Physiology of the Eye, 10th edn. Mosby: St. Louis, MO, 2003, pp 195–233.
Glasser A, Croft MA, Brumback L, Kaufman PL . Ultrasound biomicroscopy of the aging rhesus monkey ciliary region. Optom Vis Sci 2001; 78: 417–424.
Glasser A, Kaufman PL . The mechanism of accommodation in primates. Ophthalmology 1999; 106: 863–872.
Strenk SA, Semmlow JL, Strenk LM, Munoz P, Gronlund-Jacob J, DeMarco JK . Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Invest Ophthalmol Vis Sci 1999; 40: 1162–1169.
Arakawa A, Tamai M . Ultrasound biomicroscopic analysis of the human ciliary body after 1 and 2% pilocarpine instillation. Ophthalmologica 2000; 214: 253–259.
Wallman J, Winawer J . Homeostasis of eye growth and the question of myopia. Neuron 2004; 43: 447–468.
Hung GK, Ciuffreda KJ . A unifying theory of refractive error development. Bull Math Biol 2000; 62: 1087–1108.
Irving EL, Sivak JG, Callender MG . Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt 1992; 12: 448–456.
Smith EL, Hung LF . The role of optical defocus in regulating refractive development in infant monkeys. Vision Res 1999; 39: 1415–1435.
Hung LF, Wallman J, Smith EL . Vision-dependent changes in the choroidal thickness of macaque monkeys. Invest Ophthalmol Vis Sci 2000; 41: 1259–1269.
Tamm E, Lutjen–Drecoll E, Jungkunz W, Rohen JW . Posterior attachment of ciliary muscle in young, accommodating old, presbyopic monkeys. Invest Ophthalmol Vis Sci 1991; 32: 1678–1692.
Jones LA, Mitchell GL, Mutti DO, Hayes JR, Moeschberger ML, Zadnik K . Comparison of ocular component growth curves among refractive error groups in children. Invest Ophthalmol Vis Sci 2005; 46: 2317–2327.
Bacskulin A, Gast R, Bergmann U, Guthoff R . Ultrasound biomicroscopy imaging of accommodative configuration changes in the presbyopic ciliary body. Ophthalmologe 1996; 93: 199–203.
Marchini G, Pagliarusco A, Toscano A, Tosi R, Brunelli C, Bonomi L . Ultrasound biomicroscopic and conventional ultrasonographic study of ocular dimensions in primary angle-closure glaucoma. Ophthalmology 1998; 105: 2091–2098.
Trindade F, Pereira F, Cronemberger S . Ultrasound biomicroscopic imaging of posterior chamber phakic intraocular lens. J Refract Surg 1998; 14: 497–503.
Castanheira VRC, Zanoto A, Malta RFS, Betinjanea J . Ultrasound biomicroscopic (UBM) quantitative analysis of ocular changes during accommodation. Invest Ophthalmol Vis Sci 2000; 41: S564.
Goldschmidt E, Fledelius H . High myopia progression and visual impairment in a nonselected group of Danish 14-year-olds followed over 40 years. Optom Vis Sci 2005; 82: 239–243.
Weiss AH . Unilateral high myopia: optical components, associated factors, and visual outcomes. Br J Ophthalmol 2003; 87: 1025–1031.
Pollard ZF, Manley D . Long-term results in the treatment of unilateral high myopia with amblyopia. Am J Ophthalmol 1974; 78: 397–399.
Sanfillipo S, Muchnick RS, Schlossman A . Visual acuity and binocularity in unilateral high myopia. Am J Ophthalmol 1980; 90: 553–557.
Rosenthal AR, Von Noorden GK . Clinical findings and therapy in unilateral high myopia associated with amblyopia. Am J Ophthalmol 1971; 78: 397–399.
Nucci P, Drack AV . Refractive surgery for unilateral high myopia in children. J AAPOS 2001; 5: 348–351.
Lesueur L, Arne JL . Phakic intraocular lens to correct high myopic amblyopia in children. J Refract Surg 2002; 18: 519–523.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial interest: None
This article was presented in part at American Society of Cataract Refractive Surgery—Symposium on Cataract, IOL, and Refractive Surgery, 27 April–2 May 2007, San Diego, CA, USA
Rights and permissions
About this article
Cite this article
Muftuoglu, O., Hosal, B. & Zilelioglu, G. Ciliary body thickness in unilateral high axial myopia. Eye 23, 1176–1181 (2009). https://doi.org/10.1038/eye.2008.178
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2008.178
Keywords
This article is cited by
-
Clinical and genetic risk factors underlying severe consequence identified in 75 families with unilateral high myopia
Journal of Translational Medicine (2024)
-
Assessing accommodative presbyopic biometric changes of the entire anterior segment using single swept-source OCT image acquisitions
Eye (2022)
-
Ciliary muscle morphology and accommodative lag in hyperopic anisometropic children
International Ophthalmology (2020)
-
Does anisometropia affect the ciliary muscle thickness? An ultrasound biomicroscopy study
International Ophthalmology (2020)
-
Glaukombehandlung bei hoher Myopie
Der Ophthalmologe (2019)