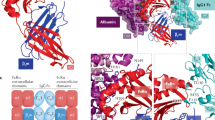
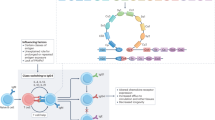
Abstract
Circulating immunoglobulin M (IgM) exists in a pentameric form, possessing a polyreactive nature that responds not only to foreign antigens but also to autoantigens; thus, it is involved in both beneficial and detrimental immune responses, including protection from infection and the progression of autoimmunity. On the other hand, IgM also behaves as a carrier of the apoptosis inhibitor of macrophage (AIM) protein, storing a large amount of the inactivated form of AIM in the blood through this association. Under different disease conditions, AIM can dissociate from IgM locally or systemically to exert its function, inducing the removal of various biological debris such as excess fat, bacteria, cancer cells or dead cell debris. Most typically, upon induction of acute kidney injury (AKI), IgM-free AIM is filtered by the glomerulus in the kidney, which stimulates the clearance of intraluminal dead cells debris at the obstructed proximal tubules, thereby facilitating the repair of kidney injury. Interestingly, cats exhibit a deficiency in AIM release from IgM, which may increase their susceptibility to renal failure. Conversely, association with AIM inhibits IgM binding to the Fcα/μ receptor on follicular dendritic cells at the splenic germinal center, thereby protecting the IgM immune complex from Fcα/μ receptor-mediated internalization, which supports IgM-dependent antigen presentation to B cells and stimulates high-affinity IgG antibody production. The regulation of AIM–IgM binding, resulting from the discovery of reciprocal actions between AIM and IgM, could lead to the development of novel therapies against different diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Miyazaki T, Hirokami Y, Matsuhashi N, Takatsuka H, Naito M. Increased susceptibility of thymocytes to apoptosis in mice lacking AIM, a novel murine macrophage-derived soluble factor belonging to the scavenger receptor cysteine-rich domain superfamily. J Exp Med 1999; 189: 413–422.
Arai S, Maehara N, Iwamura Y, Honda S, Nakashima K, Kai T et al. Obesity-associated autoantibody production requires AIM to retain IgM immune complex on follicular dendritic cells. Cell Rep 2013; 3: 1187–1198.
Yamazaki T, Mori M, Arai S, Tateishi R, Abe M, Ban M et al. Circulating AIM as an indicator of liver damage and hepatocellular carcinoma in humans. PLoS One 2014; 9: e109123.
Resnick D, Pearson A, Krieger M. The SRCR superfamily: a family reminiscent of the Ig superfamily. Trends Biochem Sci 1994; 19: 5–8.
Gebe JA, Kiener PA, Ring HZ, Li X, Francke U, Aruffo A. Molecular cloning, mapping to human chromosome 1 q21-q23, and cell binding characteristics of Spalpha, a new member of the scavenger receptor cysteine-rich (SRCR) family of proteins. J Biol Chem 1997; 272: 6151–6158.
Mori M, Kimura H, Iwamura Y, Arai S, Miyazaki T. Modification of N-glycosylation modulates the secretion and lipolytic function of apoptosis inhibitor of macrophage (AIM). FEBS Lett 2012; 586: 3569–3574.
Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell 2004; 119: 299–309.
Valledor AF, Hsu LC, Ogawa S, Sawka-Verhelle D, Karin M, Glass CK. Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc Natl Acad Sci USA 2004; 101: 17813–17818.
Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL et al. A role of the apoptosis inhibitory factor AIM/Sp/Api6 in atherosclerosis development. Cell Metab 2005; 1: 201–213.
Hamada M, Nakamura M, Tran MT, Moriguchi T, Hong C, Ohsumi T et al. MafB promotes atherosclerosis by inhibiting foam-cell apoptosis. Nat Commun 2014; 5: 3147.
Tissot JD, Sanchez JC, Vuadens F, Scherl A, Schifferli JA, Hochstrasser DF et al. IgM are associated to Sp alpha (CD5 antigen-like). Electrophoresis 2002; 23: 1203–1206.
Kai T, Yamazaki T, Arai S, Miyazaki T. Stabilization and augmentation of circulating AIM in mice by synthesized IgM-Fc. PLoS One 2014; 9: e97037.
Erlandsson L, Andersson K, Sigvardsson M, Lycke N, Leanderson T. Mice with an inactivated joining chain locus have perturbed IgM secretion. Eur J Immunol 1998; 28: 2355–2365.
Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol 1998; 160: 4776–4787.
Yamazaki S, Sugisawa R, Hiramoto E, Takai R, Matsumoto A, Senda Y et al. A proteolytic modification of AIM promotes its renal excretion. Sci Rep 2016; 6: 38762.
Kurokawa J, Arai S, Nakashima K, Nishijima A, Miyake K, Ose R et al. AIM is endocytosed into adipocytes and decreases lipid droplets via inhibition of fatty acid synthase activity. Cell Metab 2010; 11: 479–492.
Iwamura Y, Mori M, Nakashima K, Mikami T, Murayama K, Arai S et al. Apoptosis inhibitor of macrophage (AIM) diminishes lipid droplet-coating proteins leading to lipolysis in adipocytes. Biochem Biophys Res Commun 2012; 422: 476–481.
Maehara N, Arai S, Mori M, Iwamura Y, Kurokawa J, Kai T et al. Circulating AIM prevents hepatocellular carcinoma through complement activation. Cell Rep 2014; 9: 61–74.
Polo S, Pece S, Di Fiore PP. Endocytosis and cancer. Curr Opin Cell Biol 2004; 16: 156–161.
Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer 2008; 8: 835–850.
Ozawa T, Maehara N, Kai T, Arai S, Miyazaki T. Dietary fructose-induced hepatocellular carcinoma development manifested in mice lacking apoptosis inhibitor of macrophage (AIM). Genes Cells 2016; 21: 1320–1332.
Vera J, Fenutría R, Cañadas O, Figueras M, Mota R, Sarrias MR et al. The CD5 ectodomain interacts with conserved fungal cell wall components and protects from zymosan-induced septic shock-like syndrome. Proc Natl Acad Sci USA 2009; 106: 1506–1511.
Miyazaki T, Arai S. A defense system against multiple diseases via biological garbage clearance mediated by soluble scavenger proteins. Inflamm Regen 2015; 35: 203–209.
Henson PM, Vandivier RW, Douglas IS. Cell death, remodeling, and repair in chronic obstructive pulmonary disease? Proc Am Thorac Soc 2006; 3: 713–717.
Sandahl M, Hunter DM, Strunk KE, Earp HS, Cook RS. Epithelial cell-directed efferocytosis in the post-partum mammary gland is necessary for tissue homeostasis and future lactation. BMC Dev Biol 2010; 10: 122.
Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter-Hufford A, Borish L et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature 2012; 493: 547–551.
Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 2013; 113: 1004–1012.
Mochizuki A, Pace A, Rockwell CE, Roth KJ, Chow A, O'Brien KM et al. Hepatic stellate cells orchestrate clearance of necrotic cells in a hypoxia-inducible factor-1α-dependent manner by modulating macrophage phenotype in mice. J Immunol 2014; 192: 3847–3857.
Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int 2009; 76: 1089–1097.
Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 2009; 20: 223–228.
Bedford M, Farmer C, Levin A, Ali T, Stevens P. Acute kidney injury and CKD: chicken or egg? Am J Kidney Dis 2012; 59: 485–491.
Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013; 8: 1482–1493.
Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest 2014; 124: 2355–2363.
Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2008; 2: 284–291.
Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 2011; 121: 4210–4221.
Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci USA 2014; 111: 1527–1532.
Arai S, Kitada K, Yamazaki T, Takai R, Zhang X, Tsugawa Y et al. Apoptosis inhibitor of macrophage protein enhances intraluminal debris clearance and ameliorates acute kidney injury in mice. Nat Med 2016; 22: 183–193.
The American Veterinary Medical Foundation. Total Pet Ownership and Pet Populations. US Pet Ownership and Demographics Sourcebook, Section 1, 2012, American Veterinary Medical Association: Schaumburg, IL, USA..
The European Pet Food Industry.Facts and Figures, 2014. Available at: http://www.fediaf.org/fileadmin/user_upload/Secretariat/facts_and_figures_2014.pdf.
Lulich JP, O’Brien TD, Osborne CA, Polzin DJ. Feline renal failure: questions, answers, questions. Compend Contin Educ Pract Vet 1992; 14: 127–152.
Brown SA. Linking treatment to staging in chronic kidney disease. Consultations in Feline Internal Medicine (edit. By August JR) 2010; 6: 475–482.
White JD, Norris JM, Baral RM, Malik R. Naturally-occurring chronic renal disease in Australian cats: a prospective study of 184 cases. Aust Vet J 2006; 84: 188–194.
White JD, Malik R, Norris JM. Feline chronic kidney disease: can we move from treatment to prevention? Vet J 2011; 190: 317–322.
Sugisawa S, Hiramoto E, Matsuoka M, Iwai S, Takai R, Yamazaki T et al. Impact of feline AIM on the susceptibility of cats to renal disease. Sci Rep 2016; 6: 35251.
Schmiedt CW, Brainard BM, Hinson W, Brown SA, Brown CA. Unilateral renal ischemia as a model of acute kidney injury and renal fibrosis in cats. Vet Pathol 2016; 53: 87–101.
Gadjeva MG, Rouseva MM, Zlatarova AS, Reid KB, Kishore U, Kojouharova MS. Interaction of human C1q with IgG and IgM: revisited. Biochemistry 2008; 47: 13093–13102.
Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol 1993; 11: 501–538.
Hardy RR, Hayakawa K. CD5 B cells, a fetal B cell lineage. Adv Immunol 1994; 55: 297–339.
Boes M. Role of natural and immune IgM antibodies in immune responses. Mol Immunol 2000; 37: 1141–1149.
Pepys MB. Role of complement in the induction of immunological responses. Transplant Rev 1976; 32: 93–120.
Ahearn JM, Fearon DT. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21). Adv Immunol 1989; 46: 183–219.
Heyman B. The immune complex: possible ways of regulating the antibody response. Immunol Today 1990; 11: 310–313.
Carroll MC. The role of complement and complement receptors in induction and regulation of immunity. Annu Rev Immunol 1998; 16: 545–568.
Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol 2008; 20: 14–25.
Honda S, Kurita N, Miyamoto A, Cho Y, Usui K, Takeshita K et al. Enhanced humoral immune responses against T-independent antigens in Fc alpha/muR-deficient mice. Proc Natl Acad Sci USA 2009; 106: 11230–11235.
Shibuya A, Sakamoto N, Shimizu Y, Shibuya K, Osawa M, Hiroyama T et al. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat Immunol 2000; 1: 441–446.
Kojima A, Iwata K, Seya T, Matsumoto M, Ariga H, Atkinson JP et al. Membrane cofactor protein (CD46) protects cells predominantly from alternative complement pathway-mediated C3-fragment deposition and cytolysis. J Immunol 1993; 151: 1519–1527.
Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med 1984; 160: 1558–1578.
Miwa T, Song WC. Membrane complement regulatory proteins: insight from animal studies and relevance to human diseases. Int Immunopharmacol 2001; 1: 445–459.
Rosenbloom AL. Obesity, insulin resistance, beta-cell autoimmunity, and the changing clinical epidemiology of childhood diabetes. Diabetes Care 2003; 26: 2954–2956.
Hersoug LG, Linneberg A. The link between the epidemics of obesity and allergic diseases: does obesity induce decreased immune tolerance? Allergy 2007; 62: 1205–1213.
Cambuli VM, Incani M, Cossu E, Congiu T, Scano F, Pilia S et al. Prevalence of type 1 diabetes autoantibodies (GADA, IA2, and IAA) in overweight and obese children. Diabetes Care 2010; 33: 820–822.
Marzullo P, Minocci A, Tagliaferri MA, Guzzaloni G, Di Blasio A, De Medici C et al. Investigations of thyroid hormones and antibodies in obesity: leptin levels are associated with thyroid autoimmunity independent of bioanthropometric, hormonal, and weight-related determinants. J Clin Endocrinol Metab 2010; 95: 3965–3972.
Badaru A, Pihoker C. Type 2 diabetes in childhood: clinical characteristics and role of β-cell autoimmunity. Curr Diab Rep 2012; 12: 75–81.
Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med 2011; 217: 610–617.
MacLennan IC, Gray D, Kumararatne DS, Bazin H. The lymphocytes of splenic marginal zones: a distinct B-cell lineage. Immunol Today 1982; 3: 305–307.
Lopes-Carvalho T, Kearney JF. Development and selection of marginal zone B cells. Immunol Rev 2004; 197: 192–205.
Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu Rev Immunol 2005; 23: 161–196.
Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol 1999; 162: 7198–7207.
Meyer-Bahlburg A, Bandaranayake AD, Andrews SF, Rawlings DJ. Reduced c-myc expression levels limit follicular mature B cell cycling in response to TLR signals. J Immunol 2009; 182: 4065–4075.
Li QZ, Xie C, Wu T, Mackay M, Aranow C, Putterman C et al. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J Clin Invest 2005; 115: 3428–3439.
Li QZ, Zhou J, Wandstrat AE, Carr-Johnson F, Branch V, Karp DR et al. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol 2007; 147: 60–70.
Li QZ, Zhou J, Lian Y, Zhang B, Branch VK, Carr-Johnson F et al. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin Exp Immunol 2010; 159: 281–291.
Underhill GH, Minges-Wols HA, Fornek JL, Witte PL, Kansas GS. IgG plasma cells display a unique spectrum of leukocyte adhesion and homing molecules. Blood 2002; 99: 2905–2912.
Halliwell RE. Autoimmune diseases in domestic animals. J Am Vet Med Assoc 1982; 181: 1088–1096.
Martínez VG, Moestrup SK, Holmskov U, Mollenhauer J, Lozano F. The conserved scavenger receptor cysteine-rich superfamily in therapy and diagnosis. Pharmacol Rev 2011; 63: 967–1000.
Sanjurjo L, Aran G, Roher N, Valledor AF, Sarrias MR. AIM/CD5L: a key protein in the control of immune homeostasis and inflammatory disease. J Leukoc Biol 2015; 98: 173–184.
Miyazaki T, Kurokawa J, Arai S. AIMing at metabolic syndrome—towards the development of novel therapies for metabolic diseases via apoptosis inhibitor of macrophage (AIM). Circ J 2011; 75: 2522–2531.
Shuai Z, Wang J, Badamagunta M, Choi J, Yang G, Zhang W et al. The fingerprint of antimitochondrial antibodies and the etiology of primary biliary cholangitis. Hepatology 2017; 65: 1670–1682.
Hisamoto S, Shimoda S, Harada K, Iwasaka S, Onohara S, Chong Y et al. Hydrophobic bile acids suppress expression of AE2 in biliary epithelial cells and induce bile duct inflammation in primary biliary cholangitis. J Autoimmun 2016; 75: 150–160.
Acknowledgements
This work was supported by AMED-CREST, Japan Agency for Medical Research Development (to TM), a MEXT Grant-in-Aid for Scientific Research (S) Grant number 16H06389 (to TM) and (B) Grant number 16H05313 (to SA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Miyazaki, T., Yamazaki, T., Sugisawa, R. et al. AIM associated with the IgM pentamer: attackers on stand-by at aircraft carrier. Cell Mol Immunol 15, 563–574 (2018). https://doi.org/10.1038/cmi.2017.141
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2017.141
This article is cited by
-
The effects of apoptosis inhibitor of macrophage in kidney diseases
European Journal of Medical Research (2024)
-
Secreted IgM modulates IL-10 expression in B cells
Nature Communications (2024)
-
Intravenous IgM-enriched immunoglobulins in critical COVID-19: a multicentre propensity-weighted cohort study
Critical Care (2022)
-
Limited proteolysis–mass spectrometry reveals aging-associated changes in cerebrospinal fluid protein abundances and structures
Nature Aging (2022)
-
Hepatocellular carcinoma diagnosis using a novel electrochemiluminescence immunoassay targeting serum IgM-free AIM
Clinical Journal of Gastroenterology (2022)