Abstract
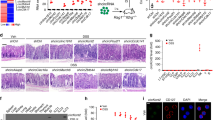
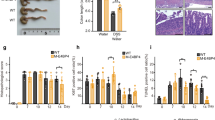
Sustained inflammation from infiltrated immune cells plays a pivotal role in the pathogenesis of ulcerative colitis (UC). Previously, we established the role of ribosomal protein L13a in the regulation of an inflammation-responsive post-transcriptional operon in myeloid cells. However, the role of this protein as a molecular cue to control the severity of colitis is not known. Here, we examined whether L13a-dependent translational control in macrophages could serve as an endogenous defense against colitis. The administration of dextran sodium sulfate induced experimental colitis in myeloid-specific L13a-knockout (KO) and control mice. Pathological scoring and injury to the colon mucosa evaluated the severity of colitis. The steady-state levels of several pro-inflammatory cytokines and chemokines were determined through ELISA and polyribosome profile analysis. Rapid weight loss, severe rectal bleeding, shortening of the colon, and significantly reduced survival rate were observed in the KO mice. Histopathological analysis of the colons of KO mice showed a severe disruption of epithelial crypts with immune cell infiltrates. Elevated levels of several inflammatory cytokines and chemokines and abrogation of their naturally imposed translational silencing were observed in the colons of the KO mice. Higher serum levels of several pro-inflammatory cytokines and the release of gut bacteria and endotoxins into the blood streams of KO mice were detected, suggesting the amplification of the inflammatory response to septicemia. Taken together, these results reveal an essential role for L13a in the endogenous protection against UC and demonstrate the potential for new therapeutic opportunities through the deliberate promotion of this mechanism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Danese S, Fiocchi C . Ulcerative colitis. N Engl J Med 2011; 365: 1713–1725.
Heinsbroek SE, Gordon S . The role of macrophages in inflammatory bowel diseases. Expert Rev Mol Med 2009; 11: e14.
Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 2012; 37: 1076–1090.
Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012; 491: 119–124.
Xavier RJ, Podolsky DK . Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007; 448: 427–434.
Neurath MF . Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014; 14: 329–342.
Kamada N, Seo SU, Chen GY, Nunez G . Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 2013; 13: 321–335.
Mazumder B, Fox PL . Delayed translational silencing of ceruloplasmin transcript in gamma interferon-activated U937 monocytic cells: role of the 3′ untranslated region. Mol Cell Biol 1999; 19: 6898–6905.
Mazumder B, Sampath P, Seshadri V, Maitra RK, DiCorleto PE, Fox PL . Regulated release of L13a from the 60S ribosomal subunit as a mechanism of transcript-specific translational control. Cell 2003; 115: 187–198.
Sampath P, Mazumder B, Seshadri V, Gerber CA, Chavatte L, Kinter M et al. Noncanonical function of glutamyl-prolyl-tRNA synthetase: gene-specific silencing of translation. Cell 2004; 119: 195–208.
Chaudhuri S, Vyas K, Kapasi P, Komar AA, Dinman JD, Barik S et al. Human ribosomal protein L13a is dispensable for canonical ribosome function but indispensable for efficient rRNA methylation. RNA 2007; 13: 2224–2237.
Kapasi P, Chaudhuri S, Vyas K, Baus D, Komar AA, Fox PL et al. L13a blocks 48S assembly: role of a general initiation factor in mRNA-specific translational control. Mol Cell 2007; 25: 113–126.
Vyas K, Chaudhuri S, Leaman DW, Komar AA, Musiyenko A, Barik S et al. Genome-wide polysome profiling reveals an inflammation-responsive posttranscriptional operon in gamma interferon-activated monocytes. Mol Cell Biol 2009; 29: 458–470.
Mazumder B, Li X, Barik S . Translation control: a multifaceted regulator of inflammatory response. J Immunol 2010; 184: 3311–3319.
Poddar D, Basu A, Baldwin WM, 3rd, Kondratov RV, Barik S, Mazumder B . An extraribosomal function of ribosomal protein L13a in macrophages resolves inflammation. J Immunol 2013; 190: 3600–3612.
Basu A, Poddar D, Robinet P, Smith JD, Febbraio M, Baldwin WM, 3rd et al. Ribosomal protein L13a deficiency in macrophages promotes atherosclerosis by limiting translation control-dependent retardation of inflammation. Arterioscler Thromb Vasc Biol 2014; 34: 533–542.
Sampath P, Mazumder B, Seshadri V, Fox PL . Transcript-selective translational silencing by gamma interferon is directed by a novel structural element in the ceruloplasmin mRNA 3′ untranslated region. Mol Cell Biol 2003; 23: 1509–1519.
Elson CO, Sartor RB, Tennyson GS, Riddell RH . Experimental models of inflammatory bowel disease. Gastroenterology 1995; 109: 1344–1367.
Perse M, Cerar A . Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol 2012; 2012: 718617.
Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R . A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990; 98: 694–702.
Geem D, Medina-Contreras O, Kim W, Huang CS, Denning TL . Isolation and characterization of dendritic cells and macrophages from the mouse intestine. J Vis Exp 2012; 63: e4040.
Platt AM, Bain CC, Bordon Y, Sester DP, Mowat AM . An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. J Immunol 2010; 184: 6843–6854.
Medina-Contreras O, Geem D, Laur O, Williams IR, Lira SA, Nusrat A et al. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest 2011; 121: 4787–4795.
Berndt BE, Zhang M, Chen GH, Huffnagle GB, Kao JY . The role of dendritic cells in the development of acute dextran sulfate sodium colitis. J Immunol 2007; 179: 6255–6262.
Morteau O, Morham SG, Sellon R, Dieleman LA, Langenbach R, Smithies O et al. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J Clin Invest 2000; 105: 469–478.
Da Silva AP, Pollett A, Rittling SR, Denhardt DT, Sodek J, Zohar R . Exacerbated tissue destruction in DSS-induced acute colitis of OPN-null mice is associated with downregulation of TNF-alpha expression and non-programmed cell death. J Cell Physiol 2006; 208: 629–639.
Lee SH, Starkey PM, Gordon S . Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J Exp Med 1985; 161: 475–489.
Zigmond E, Jung S . Intestinal macrophages: well educated exceptions from the rule. Trends Immunol 2013; 34: 162–168.
Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 2014; 15: 929–937.
Atreya R, Neurath MF . Chemokines in inflammatory bowel diseases. Dig Dis 2010; 28: 386–394.
Danese S, Gasbarrini A . Chemokines in inflammatory bowel disease. J Clin Pathol 2005; 58: 1025–1027.
Ajuebor MN, Kunkel SL, Hogaboam CM . The role of CCL3/macrophage inflammatory protein-1alpha in experimental colitis. Eur J Pharmacol 2004; 497: 343–349.
Waddell A, Ahrens R, Tsai YT, Sherrill JD, Denson LA, Steinbrecher KA et al. Intestinal CCL11 and eosinophilic inflammation is regulated by myeloid cell-specific RelA/p65 in mice. J Immunol 2013; 190: 4773–4785.
Melgar S, Drmotova M, Rehnstrom E, Jansson L, Michaelsson E . Local production of chemokines and prostaglandin E2 in the acute, chronic and recovery phase of murine experimental colitis. Cytokine 2006; 35: 275–283.
Peterson LW, Artis D . Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 2014; 14: 141–153.
Kim YS, Ho SB . Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 2010; 12: 319–330.
Gallo RL, Hooper LV . Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 2012; 12: 503–516.
Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC . The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 2008; 105: 15064–15069.
Piccirillo CA, Bjur E, Topisirovic I, Sonenberg N, Larsson O . Translational control of immune responses: from transcripts to translatomes. Nat Immunol 2014; 15: 503–511.
Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012; 61: 1693–1700.
Acknowledgements
The authors would like to thank Nina Dvorina for providing technical assistance with the immunohistochemistry experiments. The authors would also like to thank Dr. Patricia Stanhope Baker for assistance with manuscript editing. This work was financially supported by the National Institute of Health (NIH) Public Health Service Grant No. HL 79164 (to B. Mazumder), American Heart Association Pre-doctoral Fellowship Grant No. 11PRE7660008 and a Doctoral Dissertation Award from the Cleveland State University Office of Research (to D. Poddar), and a NIH Grant No. PO1AI087586 (to W.M. Baldwin). B. Mazumder also received financial support from the Center for Gene Regulation in Health and Disease at Cleveland State University and an Ohio Third Frontier Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information of this article can be found on Cellular & Molecular Immunology website: http://www.nature.com/cmi.
Supplementary information
Rights and permissions
About this article
Cite this article
Poddar, D., Kaur, R., Baldwin, W. et al. L13a-dependent translational control in macrophages limits the pathogenesis of colitis. Cell Mol Immunol 13, 816–827 (2016). https://doi.org/10.1038/cmi.2015.53
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2015.53
Keywords
This article is cited by
-
GAITing the GUT
Cellular & Molecular Immunology (2018)