Abstract
Members of the inhibitor of apoptosis protein (IAP) family have demonstrated functions in cell death, cell signalling, cell migration and mitosis. Several of them are E3 enzymes in the ubiquitination of proteins that leads to their degradation by the proteosomal machinery. We previously reported that one of them, cellular inhibitor of apoptosis protein-1 (c-IAP1), migrated from the nucleus to the surface of the Golgi apparatus in cells undergoing differentiation. Here, we show that c-IAP1 is a client protein of the stress protein HSP90β. In three distinct cellular models, the two proteins interact and migrate from the nucleus to the cytoplasm along the differentiation process through a leptomycin B-sensitive pathway. Inhibition of HSP90 proteins by small chemical molecules and specific depletion of HSP90β isoform by siRNA both lead to auto-ubiquitination of c-IAP1 and its degradation by the proteasome machinery. This chaperone function of HSP90 towards c-IAP1 is specific of its β isoform as specific depletion of HSP90α does not affect c-IAP1 content. Chemical inhibition of HSP90 or siRNA-mediated depletion of HSP90β both inhibit cell differentiation, which can be reproduced by siRNA-mediated depletion of c-IAP1. Altogether, these results suggest that HSP90β prevents auto-ubiquitination and degradation of its client protein c-IAP1, whose depletion would be sufficient to inhibit cell differentiation.
Similar content being viewed by others
Main
Members of the inhibitor of apoptosis protein (IAP) family were initially described as a series of natural inhibitors of cell death. Among the eight human proteins of this family, X-linked IAP (XIAP) demonstrated to be the bona fide caspase inhibitor1 whereas the others demonstrated, for the most part, functions in cell signalling2 and mitosis.3 Several of these proteins harbour a Really Interesting New Gene (RING) domain at the carboxy terminus and function as an E3 enzyme in the cascade of ubiquitination that targets proteins to the ubiquitin-proteasome degradation machinery.4
One of these IAP with a RING domain is cellular IAP1 (c-IAP1) that was initially described as a signalling molecule.2 Although c-IAP1 has subsequently been described as a direct inhibitor of caspases, this remains a controversial issue.5, 6 Due to its E3 function, c-IAP1 is responsible for the ubiquitination and subsequent degradation of the adaptor protein TNF receptor associated factor 27 and the serine–threonine apoptosis signal-regulating kinase 18 in the tumour necrosis factor alpha (TNFα) signalling pathway. In this TNFα pathway, c-IAP1 was reported also to interact with the serine-threonine kinases receptor interacting protein 2 and nuclear factor kappaB (NF-κB) essential modifier, upstream of NF-κB,9 and to block caspase-8 activation, downstream of NF-κB.10
Deletion experiments in Drosophila melanogaster have revealed other functions of IAPs in cell differentiation,11 cell migration,12 and immune response.13 In mammals, c-IAP1-deficient mice develop normally. However, cells from c-IAP1−/− mice express markedly elevated levels of its highly homologous protein c-IAP2, suggesting that a redundancy in the function of the two proteins might take place.14 Deletion of several other IAPs have demonstrated to lead to defaults in the development. XIAP deficiency delays development of the mammary gland15 whereas that of BRUCE, a giant E3 ubiquitin ligase IAP, affects placenta development16 and T-cell maturation.17 How IAPs interfere with the differentiation pathways remain undetermined.
We have reported previously that c-IAP1 was located in the nucleus of undifferentiated cells and migrated to the cytoplasm along the differentiation process to concentrate at the surface of the Golgi apparatus in terminally differentiated cells.18 In a search for a function of c-IAP1 in the different cellular compartments, we have identified an interaction between c-IAP1 and heat-shock protein 90 β isoform (HSP90β), a molecular chaperone abundant in cancer cells and whose inhibition is currently being tested in cancer therapy. HSP90β migrates with c-IAP1 from the nucleus to the cytoplasm and its inhibition induces c-IAP1 degradation by the proteasomal machinery, which can be sufficient to block the differentiation process.
Results
HSP90β associates with c-IAP1
In an approach to determine the functional role of c-IAP1 in the different cell compartments, we performed a mass spectrometry analysis of the proteins immunoprecipitated with an anti-c-IAP1 antibody in monocytic THP1 cells before (c-IAP1 located in the nucleus) and after (c-IAP1 located in the cytoplasm) 12-0-tetradecanoylphorbol-13-acetate (TPA)-induced differentiation into macrophages. Interestingly, in three different experiments, we identified peptides corresponding to the isoform β of the heat-shock protein HSP90 whereas no peptide of the α isoform was identified (Table 1). HSP90 is the most abundant molecular chaperone in eukaryotic cells, comprising ∼1–2% of cellular proteins under non-stressed conditions. In mammals, two isoforms exist, which are encoded by different but highly conserved genes.19 We confirmed the interaction of c-IAP1 with HSP90β by immunoprecipitation using an antibody that specifically recognizes the HSP90β isoform and immunoblotting with an anti-c-IAP1 antibody (Figure 1a). In contrast, immunoprecipitation with an antibody that specifically recognizes HSP90α isoform did not detect a strong interaction of this isoform with c-IAP1 (Figure 1a). FRET analysis confirmed the c-IAP1/HSP90β-interaction in THP1 cells (energy level higher than five, in green, yellow or red (Figure 1b). In these THP1 cells, c-IAP1/HSP90β-interaction was observed only in the nucleus, confirming our previous observations that c-IAP1 has a nuclear localization in undifferentiated cells.18 The c-IAP1/HSP90β-interaction was also observed in differentiated cells (Figure 1c). TPA-induced differentiation of THP1 cells was associated with an increase in c-IAP1 expression (Figure 1c, lower panel) together with an increased amount of c-IAP1 associated with HSP90β (Figure 1c, upper panel). Mutations in leucine residues that characterize the three potential c-IAP1 nuclear export sequences18, 20 did not affect the HSP90β/c-IAP1 interaction (data not shown), indicating that the protein–protein interaction involves other c-IAP1 sequences.
c-IAP1 interacts with HSP90β. (a) Immunodetection of c-IAP1, HSP90α and HSP90β after immunoprecipitation with HSP90α (IP HSP90α) or HSP90β (IP HSP90β) or a non-relevant (IP GFP) antibody (Ab) in undifferentiated THP1 cells. (b) FRET analysis of c-IAP1 interaction with HSP90β in undifferentiated THP1 cells. (c) Upper blot, immunodetection of c-IAP1 after immunoprecipitation with HSP90β Ab (IP HSP90β) in THP1 cells left untreated or treated with TPA (20 nM, 24 h). Immunoprecipitations with HSP27 or GFP antibodies were used as negative controls (IP HSP27, IP GFP). Lower blot, amount of c-IAP1 in the inputs. HSC70 was used as protein loading control
HSP90β translocates with c-IAP1 from the nucleus to the cytoplasm
To determine whether HSP90β relocalized with c-IAP1 from the nucleus to the cytosol during differentiation, we performed immunofluorescence and cell fractionation studies. Immunofluorescence analysis in TPA-differentiated compared to undifferentiated THP1 cells indicated that c-IAP1 and HSP90β both translocated from the nucleus to the cytosol (Figure 2a). The cellular redistribution of the two proteins was also observed in two other cell models. Primary monocytes (peripheral blood monocytic cells) were induced to differentiate into macrophages upon M-CSF exposure for 3 days and their differentiation was assessed by morphological changes and the expression of CD71 and CD163 at their surface (Figure 2a and Supplementary Figure 1). HT-29 colon cancer cells were induced to differentiate in mucin-producing cells by exposure to sodium butyrate (NaB) and their differentiation was assessed by morphological changes and the expression of villin (Figure 2a). In all cases, the differentiation associated nuclear extrusion of both c-IAP1 and HSP90β was blocked by cell treatment with leptomycin B, a specific inhibitor of the nuclear export protein exportin 1 (Supplementary Figure 2).18, 20 The nuclear exit of c-IAP1 and HSP90β seems to be an early event in a differentiation process. Only one hour after TPA treatment of THP1 cells, the content of both proteins in the nucleus was strongly decreased (Figure 2b).
Nuclear extrusion of c-IAP1 and HSP90β during cell differentiation. (a) Fluorescence microscopy analysis of HSP90β (red) and c-IAP1 (green) in monocytic cells (THP1 and peripheral blood mononuclear cells) and colon cancer HT-29 cells. Cells were either left untreated (control) or exposed to TPA (20 nM, 24 h), M-CSF (100 ng/ml, 3 days) or NaB (3 mM, 3 days), respectively. Differentiation was monitored by flow cytometric analysis of CD11b or CD71 membrane expression for THP1 and primary monocytes respectively, and by immunodetection of villin for HT-29 cells. HSC70 was used as protein loading control. Nuclei, labelled with Hoescht 33342, are stained in blue. Magnification × 300. One representative figure is shown (N=4). (b) Western blot analysis of c-IAP1 and HSP90β in nuclear extracts of THP1 cells, either left untreated or treated with 20 nM TPA for 1, 2 or 8 h. HSC70 was used as protein loading control
c-IAP1 is a client protein of HSP90
Heat shock protein 90 is required for the maturation and functional stability of a number of proteins termed HSP90 client proteins. Upon inhibition of HSP90, the client protein is not anymore chaperoned and, as a consequence, is degraded by the proteasome. Since c-IAP1 associated with HSP90β and these two proteins were redistributed from the nucleus to the cytoplasm with the same kinetics, we wondered whether c-IAP1 was a client protein of HSP90β, that is, was degraded by the proteasome machinery when HSP90 activity was inhibited. To answer this question, we used three small soluble inhibitors of HSP90, including the benzoquinone ansamycin 17-allylaminogeldanamycin (17-AAG) and the synthetic small molecule inhibitors of the purine scaffold class PU-H71 and PU-DZ8. Exposure of HT-29 cells for 18 h to any of the three compounds efficiently decreased the expression of the protein RIP1, a well known HSP90 client protein (Figure 3a),21 in the absence of any significant apoptosis (not shown). These treatments all reduced the amount of the endogenous c-IAP1 detected with a virtually complete disappearance of the protein after 24 h of drug treatment, as demonstrated by western blot (Figure 3b) and immunofluorescence experiments (Supplementary Figure 3). The pattern of c-IAP1 levels after the different treatments was similar to that of RIP1 (Figure 3b). The disappearance of c-IAP1, as that of RIP1, was prevented by cotreatment with the proteasome inhibitor MG132 whose efficacy was assessed by quantifying the ability of cell lysates to cleave the substrate Suc-LLVY-AMC22 (Figure 3b) and by determining the total amount of ubiquitinated proteins in the cells (Supplementary Figure 4). As expected, this total amount of ubiquitinated proteins inversely correlated with the proteasome activity, reflecting their degradation.
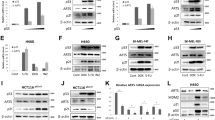
c-IAP1 is a client protein of HSP90. (a) Western blot analysis of RIP in untreated cells (control) or treated for 18 h with 17-AAG (1 μM), PU-H71 (200 nM) or PU-DZ8 (100 nM). HSC70 was used as protein loading control. (b) HT-29 cells were treated with the HSP90 inhibitors 17-AAG (1 μM), PU-H71 (200 nM) or PU-DZ8 (100 nM) for 18 h in the presence or absence of MG132 (2 μM) before measuring the ability of cell lysates to cleave the substrate Suc-LLVY-AMC. c-IAP1 and RIP1 protein contents were determined by western blot. HSC70 was used as protein loading control. (c) Upper panel, HT-29 control transfected (Control), transfected with a c-IAP1 wild-type construct (GFP c-IAP1 wt) or transfected with a c-IAP mutant in which the RING domain has been deleted (GFP c-IAP1ΔRING), were either left untreated or treated with 17-AAG (1 μM, 18 h) in the presence or absence of MG132 (2 μM, 18 h). The amount of the c-IAP1 was immunodetected (IB) with a c-IAP1 Ab or GFP Ab (for endogen and transfected c-IAP1 respectively) after immunoprecipitation with an ubiquitin Ab (IP Ub). Lower panel, immunodetection of c-IAP1 (endogen and GFP-derived constructs) in the previously described transfected cells, before ubiquitin immunoprecipitation (inputs). HSC70 was used as protein loading control. (d) Immunodetection of c-IAP1, HSP90α and HSP90β in HT-29 cells, transfected with scramble siRNA or transfected with a siRNA that targets HSP90α or HSP90β respectively. HSC70 was used as protein loading control
Immunoprecipitation of ubiquitinated proteins followed by identification of c-IAP1 by immunoblotting indicated that ubiquitinated c-IAP1 was detected upon treatment with 17-AAG in the presence of the proteasome inhibitor MG132 (Figure 3c). Interestingly, deletion of the RING domain of c-IAP1 prevented its ubiquitination upon inhibition of HSP90 by 17-AAG, suggesting its auto-ubiquitination (Figure 3c).23 HSP90 inhibitors equally target HSP90α and HSP90β isoforms. To determine which of the two isoforms was involved in the degradation of c-IAP1, we depleted one or the other isoform by the use of specific siRNAs. As shown in Figure 3d, depletion of HSP90β induced a decrease in c-IAP1 expression while no effect was observed when depleting HSP90α (Figure 3d).
Inhibition of HSP90 blocks cells differentiation
Exposure of THP1 cells to the three tested HSP90 inhibitors was observed to prevent their TPA-induced differentiation, as assessed by the lack of characteristic changes in cell morphology (not shown) and the lack of CD11b expression increase at the cell surface (Figure 4a). Similarly, these inhibitors prevented M-CSF-induced differentiation of peripheral blood monocytes, as indicated by the lack of cell adhesion to the culture flask (not shown) and the lack of appearance of the differentiation markers CD71 (Figure 4b) and CD163 (Supplementary Figure 1). HSP90 inhibitors also blocked the NaB-induced differentiation of HT-29 cells, as indicated by the lack of induction of villin expression (Figure 4c). siRNA-mediated decrease in the expression of HSP90β also inhibited NaB-induced villin expression in HT-29 cells whereas siRNA-mediated decrease in the expression of HSP90α did not (Figure 4d). These results suggested a specific function for HSP90β isoform in cell differentiation.
Inhibitors of HSP90 or specific depletion of HSP90β block cell differentiation. (a) Flow cytometry analysis of CD11b membrane expression in THP1 cells left untreated (control) or treated with the inhibitors of HSP90 17-AAG (1 μM), PU-H71 (200 nM) or PU-DZ8 (100 nM), in the presence or absence of TPA (20 nM, 24 h). (b) Flow cytometry analysis of CD71 membrane expression in primary monocytes before (control) and after exposure to M-CSF (100 ng/ml) for 3 days, in the presence or absence of 17-AAG (1 μM), PU-H71 (200 nM) or PU-DZ8 (100 nM). (c) Immunodetection of villin in HT-29 cells either left untreated or treated with sodium butyrate (NaB, 3 mM for 3 days), in the presence or absence of 17-AAG (1 μM), PU-H71 (200 nM) or PU-DZ8 (100 nM). HSC70 was used as protein loading control. (d) Immunodetection of Villin, HSP90α and HSP90β in HT-29 cells non transfected (control), transfected with scramble siRNA, a siRNA that targets HSP90α or a siRNA that targets HSP90β. HSC70 was used as protein loading control
c-IAP1 depletion inhibits macrophage and epithelial cells' differentiation
Since we had observed that c-IAP1 was a client protein of HSP90β, that is, that c-IAP1 was degraded by the proteasome machinery in the absence of HSP90β, we tested the effects of c-IAP1 depletion on cell differentiation. Western blots, flow cytometry and microscopy analyses showed that siRNA-mediated depletion of c-IAP1 prevented the differentiation of THP1 cells into macrophages upon TPA exposure, as assessed by the lack of morphological changes and the lack of increase in CD11b expression at the cell surface (Figure 5a). Similarly, depletion of c-IAP1 prevented the differentiation of HT-29 cells exposed to NaB, as indicated by the lack of villin expression (Figure 5b). Accordingly, a commercial inhibitor of IAPs, SMAC N7, was observed to inhibit the TPA-induced differentiation of THP1 cells (Figure 5c) and NaB-induced differentiation of HT-29 cells (Figure 5d), in a dose-dependent manner. Altogether, these results suggest that HSP90β inhibition leads to auto-ubiquitination and degradation of its client protein c-IAP1 whose depletion is sufficient to inhibit cell differentiation.
Depletion of c-IAP1 inhibits macrophage and colon cancer cells differentiation. (a) Upper panel, immunodetection of c-IAP1 in THP1 cells transfected with a pcDNA3 control construction or a c-IAP1 antisense construction (AS). Middle panel, fluorescence microscopy analysis of c-IAP1 (green) in monocytic THP1 cells transfected with a pcDNA3 control construction or a c-IAP1 antisense construct (AS), either untreated or treated with TPA (20 nM, 24 h). Lower panel, flow cytometry analysis of CD11b membrane expression in THP1 control-transfected (pcDNA) or AS-c-IAP1-transfected cells treated with TPA (20 mM, 24 h). HSC70 was used as protein loading control. (b) Upper blot, immunodetection of c-IAP1 in HT-29 cells transfected with a scramble siRNA or a siRNA that targets c-IAP1 (siRNA-cIAP1). Middle panel, fluorescence microscopy analysis of c-IAP1 (green) in HT-29 cells transfected with a scramble siRNA or a c-IAP1 targeting siRNA, then left untreated or treated with 3 mM NaB for 3 days. Lower blot, immunodetection of villin in the HT-29 cells. SC70 was used as protein loading control. (c) THP1 and HT-29 cells were pre-treated with the IAP inhibitor SMAC N7 (20 or 50 μM) before inducing their differentiation by exposure to TPA and NaB, respectively
Discussion
We and others previously reported that, during cell differentiation, c-IAP1 relocalized from the nucleus to the cytosol in an nuclear export sequences-dependent manner.18, 20 The present work demonstrates that c-IAP1 nuclear exclusion also requires the β isoform of the chaperone HSP90. We identify c-IAP1 as a client protein of HSP90β and demonstrate that the chaperone prevents the auto-ubiquitination and degradation of the IAP protein, which would otherwise block the differentiation process. Whereas deletion experiments in D. melanogaster have revealed functions of IAPs in cell differentiation,11 c-IAP1 deficient mice develop normally. This lack of phenotype was most probably related to markedly elevated levels of c-IAP2 in c-IAP1-deficient mouse cells with redundancies in the functions of the two proteins.14 Other potential explanations include the use of alternative pathways of differentiation when c-IAP1 is disrupted or the appearance of specific c-IAP1 functions in humans as compared to mice.
Interaction networks emerging from large scale experiments demonstrate that HSP90 plays a central role in multiple pathways and cellular processes.24 Some reports suggest that the two isoforms of the stress protein, HSP90α and HSP90β, might have different functions in the cells. HSP90α has often been described to protect the cells from death in stressful conditions whereas HSP90β has been involved in embryonic development, cell differentiation and cytoarchitecture maintenance.25 In D. melanogaster, homozygous point mutations of the hsp90 orthologue named hsp83 is lethal whereas heterozygous mutants are sterile.26 In Caenorhabditis elegans, deletion of daf-21, the orthologue of hsp90, affects oocyte development27 and in zebrafish, Danio rerio, disruption of hsp90 orthologue affects somatic muscle development.28 In mice, hsp90β loss-of-function is lethal, due to defects in trophoblast differentiation and placenta development that cannot be rescued by endogenous HSP90α.25 Here, we show that while HSP90β depletion blocks cell differentiation, HSP90α depletion does not seem to affect this physiological process. These results further emphasize the functional divergences between these two HSP90 proteins in humans.
HSP90 is an ubiquitous molecular chaperone that is required for the proper folding of a set of client proteins. Its broad clientele includes structurally and functionally different proteins that include a growing range of protein kinases, a variety of nuclear hormone receptors, cell surface receptors such as ErbB2/neu, transcription factors such as HIF-1α and many others.29 The molecular basis for HSP90's specificity for client proteins is largely unknown and close homologue proteins can demonstrate different dependence on HSP90 for their function. When no more chaperoned by HSP90, for example when the conformation of the stress protein changes upon binding and hydrolysis of ATP, the client protein is ubiquitinated by ligases and degraded by the proteasome.30 Here, we show that c-IAP1 is another client protein of HSP90β that protects c-IAP1 from its previously identified ability for auto-ubiquitination and degradation.31, 32
HSP90β and c-IAP1 depletion experiments reported in the present study demonstrate that both proteins are required for cell differentiation. Given the number of HSP90 client proteins in the cell, including proteins such as RIP1 and the inhibitor of NF-kB kinase (IKKα/β) that play a role in cell differentiation,33 c-IAP1 is probably not the unique mediator of HSP90 pro-differentiating effect. How c-IAP1 redistribution from the nucleus to the cytoplasm contributes to the differentiation process is still a matter of speculation. As an E3 ligase, c-IAP1 could favour the degradation of specific proteins. Caspases have been reported to be ubiquitinated by mammalian IAPs in vitro but the role of these enzymes in cell differentiation is limited to specific cell types33, 34 and evidence that IAPs physiologically ubiquitinylate caspases and promote their degradation, is still missing.32 c-IAP1 could also modulate NF-κB transcription factor activity9 or specific signalling pathways involving adaptor molecules such as TRAF27 and serine–threonine kinases such as ASK1.8
HSP90 is overexpressed in many cancers and is presumed to be required to sustain aberrant signalling in malignant cells.19 HSP90 has therefore emerged as an exciting target in cancer therapy and small molecule inhibitors of HSP90 are currently developed for clinical evaluation. Those include the three inhibitors used in this work, the 17-AAG that has completed phase I clinical trials and is currently tested in phase II trials and the purine-scaffold inhibitors PU-H71 and PU-DZ8, in phase I and advanced preclinical evaluation respectively.29 Both ansamycins and purines bind to the conserved N-terminal ATP-binding pocket at the surface of the two HSP90 proteins, HSP90α and HSP90β, which inhibits their ATP-dependent chaperone activity. It is at present unknown whether the effect of HSP90 inhibitors blocking cell differentiation has any repercussion in the treated patients. The effect of HSP90 inhibitors in the cell differentiation process has already been hinted by other authors. Geldanamycin was shown to block myoblast differentiation35 and to promote apoptosis of human leukaemic and breast cancer cells.36 The molecule was recently shown also to significantly inhibit dendritic cell functions, thereby affecting the immune response.37 However, in some circumstances and specific cellular models, HSP90 inhibitors can promote rather than inhibit cell differentiation.38 This discrepancy could be related to the preferential inhibition of HSP90α compared to HSP90β, depending on the drug and the cell type.
Altogether, our study identified an essential function of HSP90β isoform in cell differentiation, that is, to chaperone c-IAP1 and thereby protect this E3 ligase from auto-ubiquitination and degradation by the proteasomal machinery. This protective effect is essential as cell differentiation is inhibited as soon as c-IAP1 is degraded or deleted. It remains to be determined which essential function is exerted by c-IAP1 when migrating, together with HSP90β, from the nucleus to the cytoplasm in cells undergoing differentiation.
Materials and Methods
Cell culture and differentiation
THP1 monocytic leukaemia cells, obtained from DSMZ (Braunschweig, Germany), were cultured at a density up to 8 × 105 cells per ml in RPMI 1640 medium supplemented with 9% fetal bovine serum and induced to differentiate into macrophage by exposure to 12-0-tetradecanoylphorbol-13-acetate (TPA, 20 nM, Sigma-Aldrich, St. Quentin Fallavier, France) during 24 h. Cell differentiation was assessed by following morphological changes and the expression of cell surface marker CD11b-FITC by flow cytometry analysis as described.39 Monocytes from human peripheral blood were obtained with informed consent from healthy donors and purified using an isolation kit (Miltenyi Biotec, Paris, France) following manufacturer's instructions. Primary monocytes were differentiated into macrophages by treatment with macrophage colony-stimulating factor (M-CSF exposure, 100 ng/ml, 3 days, R&D Systems, Abington, UK) and differentiation was assessed by analysis of the membrane differentiation marker CD71, as described.39 The HT-29 cell line, obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA), was grown in Dulbecco's modified Eagle's medium (Sigma-Aldrich) containing 4.5 g/l of glucose and supplemented with 9% fetal bovine serum. Medium was changed every day to limit spontaneous differentiation or differentiation was induced by exposure to sodium butyrate (NaB, 3 mM, 3 days, Sigma-Aldrich) and assessed by determining the expression of villin.
HSP90 and c-IAP inhibitors
17-(Allylamino)-17-demethoxygeldanamycin (17-AAG) was purchased from BioMol (Tebu-bio, Le Perray en Yvelines, France) and used at 1 μM. PU-H71 and PU-DZ8 were provided by G Chiosis (Sloan-Kettering Cancer Center, NY) and used at 200 and 100 nM, respectively. SMAC-N7 was purchased from Calbiochem (Merck Chemicals, Nottingham, UK) and used at 20 and 50 μM during 4 h before differentiation induction of THP1 and HT-29 cells with TPA (20 nM) and NaB (3 mM) respectively.
Immunoprecipitation
Cells were lysed in immunoprecipitation buffer (50 mM HEPES, pH 7.6; 150 mM NaCl; 5 mM EDTA; 0.1% Nonidet P-40 (NP-40, Sigma-Aldrich)) during 1 h. After centrifugation during 10 min at 15 000 × g, the protein concentration of supernatant was evaluated (DC protein assay, BioRad, Marnes-la-Coquette, France) and 500 μg of protein were incubated with 2 μg of the indicated antibody (anti-GFP, anti-ubiquitin and anti-HSP90α/β from TebuBio; anti-c-IAP1 from R&D Systems; anti-HSP90α and anti-HSP90β kindly donated by D Toft (Rochester, USA)) with constant agitation at 4 °C. Then, the immuno-complexes were precipitated with protein A/G-Sepharose (Amersham, Les Ullis, France). The pellet was used for immunoblotting or MS/MS analysis.
MS/MS analysis of c-IAP1 immunoprecipitation
After Coomassie blue staining, the SDS-PAGE (polyacrylamide gel and electrophoresis) is excised in three parts. Proteins were digested in gel by trypsin at 37°C overnight. Peptides were extracted and analysed by MS/MS as described previously.40
Immunoblot analysis
Whole cell lysates were prepared by lysing the cells in immunoprecipitation buffer excepted for villin expression analysis for which proteins were extracted with CHAPS 1% (Sigma-Aldrich) diluted into Tris buffer (Tris–HCl 10 mM, pH 7.4). Cells were kept on ice for 1 h and were vortexed three times. After centrifugation (15 000 × g, 10 min, 4°C), supernatant was evaluated for protein concentration by a micro-bicinchroninic acid protein assay (Bio-Rad).
Cytoplasmic and nuclear extracts were obtained by lysing cells during 15 min on ice in a lysis buffer (10 mM Hepes, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT) with 0.6% NP-40 in the presence of protease inhibitors (Roche, Neuilly sur Seine, France). Cell lysate was centrifugated at 1200 × g for 15 min and the supernatant was carefully collected (cytoplasmic fraction). The pellet was washed once and resuspended in lysis buffer (50 mM Hepes, 150 mM NaCl, 5 mM EDTA, 0.1% NP-40, protease inhibitors) and nuclear fractions were harvested after centrifugation (15 000 × g, 10 min). Proteins were separated in SDS-PAGE onto PVDF membranes (Millipore, Molsheim, France). Immunoblot analysis was performed using specific antibodies and enhanced chemoluminescence-based detection (Millipore). The antibodies used were the rabbit polyclonal anti-HSP90β from ABR (Ozyme, St. Quentin en Yvelines, France), anti-c-IAP1 from R&D Systems, the mouse monoclonal anti-HSC70 and anti-ubiquitin from Santa-Cruz (Tebu-Bio), anti-Villin from BD Biosciences (Le Pont de Claix, France) and anti-GFP from Molecular Probes (Invitrogen, Cergy Pontoise, France). All the monoclonal antibodies used were of the IgG1 isotype.
Proteasome activity
Proteasome activity was determined as described previously.22 Briefly, cells (2 × 106 in 200 μl PBS, pH 7.4) were incubated for 30 min at 37 °C with 100 μM of the cell-permeant fluorogenic substrate N-succinyl-L-leucyl-L-leucyl-L-tyrosine-7-amido-4-methyl coumarin (Bachem, Basel, Switzerland). Fluorescence generated by its cleavage was quantified by using a Kontron SFM 25 spectrofluorometer (Kontron AG, Zurich, Switzerland). When needed, proteasome activity was inhibited by exposure of the cells to Z-leu-leu-leu-H-(aldehyde) (MG132, Peptide Institute, Osaka, Japan), at 2 μM for 18 h.
Plasmid constructs
pECFP-c-IAP1 and pEGFP-c-IAP1 plasmids were constructed by subcloning full length c-IAP1 cDNA (provided by JC Reed, La Jolla, CA, USA) into the BglII/SalI site of pECFP-C1 and pEGFP-C1 respectively (Clontech, Ozyme, St. Quentin en Yvelines, France). pEYFP-HSP90β plasmid was constructed using GFP-HSP90β provided by D Picard (Geneve, Switzerland). After PCR amplification (sense 5′-gATAGGTACCATGCCTGAGGAAGTGCAC-3′; antisense: 5′-GGTCGGATCCCTAATCGACTTCTTCCAT-3′), the cDNA encoding HSP90β was cloned into Kpn/BamH1 sites of pEYFP-C1 (Clontech). The cDNA encoding the 1-566 amino acid sequence of c-IAP1 (c-IAP1-ΔRING) was amplified by PCR from the pcDNA-myc-c-IAP1 (JC Reed, La Jolla, CA, USA) (sense: 5′-GAACAGAAACTCATCTCTGAAGAGGATCTG-3′; antisense: 5′-GATCTTTGCAACCTCCTCAATTGT-3′) and first cloned into pCR-2.1 TOPO plasmid (Invitrogen), then subcloned into the EcoR1 site of pEGFP-C2 (Clontech). The c-IAP1 antisense (AS-c-IAP1) construct was obtained by subcloning full length c-IAP1 cDNA in an antisense orientation into the EcoR1/Kpn1 site of pcDNA 3.1 (Invitrogen).
Small interfering RNA (siRNA) and transfection
HSP90α and β siRNA and scramble were purchased from Ambion (Applied Biosystems, Courtaboeuf, France) and c-IAP1 siRNA from Eurogentec (Angers, France). HT-29 cells were plated in serum-containing medium one day before transfection. Cells were transfected with siRNAs using oligofectamine (Invitrogen, Cergy-Pontoise, France) and with plasmids using Superfect transfection reagent (Qiagen, Courtaboeuf, France), following the manufacturer's instructions. Cells were analysed 48 h after transient transfection. THP1 cells were transfected by AS-c-IAP1 or pcDNA3.1, or cotransfected by pECFP-c-IAP1 and pEYFP-HSP90β using the AMAXA nucleofector kit (Amaxa, Köln, Germany). Transfected cells were selected with geneticin (0.7 μg/ml, Sigma-Aldrich).
Immunofluorescence staining
Cells were fixed in PBS-paraformaldehyde 4% during 15 min and permeabilized by incubation with PBS-Triton 0.1% for 3 min. After washing with PBS, samples were saturated with PBS-BSA 3% during 30 min before incubation overnight at 4 °C with anti-c-IAP1 (BD Biosciences) or anti-HSP90β (ABR). After four washes in PBS-BSA 1%, appropriate secondary antibodies coupled with fluorochromes (Alexa 486 and 568 nm; Molecular Probe, Leiden, Netherlands) were added during 1 h at room temperature in the dark. The nucleus was labelled by Hoescht 33342 incubation during 5 min. Images were acquired using the Cell Observer station (Zeiss, Germany). Briefly, the system is composed by an inverted microscope AxioVert 200M equipped for fluorescence with a CCD camera. All the system is motorized and controlled by the Axiovision software (Zeiss).
CFP/YFP fluorescence resonance energy transfer (FRET) analysis
For FRET imaging experiment in living cells, THP1 transfected cells in Petri dish were placed on the stage of the microscope (Cell Observer station, Zeiss). FRET image acquisition and analysis were done by the Axiovision FRET software (Zeiss) using the Youvan's calculation method.
Abbreviations
- 17-AAG:
-
17-(Allylamino)-17-demethoxygeldanamycin
- ASK1:
-
apoptosis signal-regulating kinase 1
- c-IAP1:
-
cellular IAP-1
- FBS:
-
fetal bovine serum
- FRET:
-
fluorescence resonance energy transfer
- HSP:
-
heat shock protein
- IAP:
-
inhibitor of apoptosis protein
- M-CSF:
-
macrophage colony-stimulating factor
- NaB:
-
sodium butyrate
- NEMO:
-
NF-κB essential modifier
- NES:
-
nuclear export sequence
- NF-κB:
-
nuclear factor kappaB
- NP-40:
-
Nonidet P-40
- RING:
-
really interesting new gene
- RIP:
-
receptor interacting protein
- siRNA:
-
small interfering RNA
- TNFα:
-
tumour necrosis factor alpha
- TPA:
-
12-0-tetradecanoylphorbol-13-acetate
- TRAF2:
-
TNF receptor-associated factor 2
- XIAP:
-
X-linked IAP
References
Eckelman BP, Salvesen GS, Scott FL . Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep 2006; 7: 988–994.
Werneburg BG, Zoog SJ, Dang TT, Kehry MR, Crute JJ . Molecular characterization of CD40 signaling intermediates. J Biol Chem 2001; 276: 43334–43342.
Lens SM, Vader G, Medema RH . The case for Survivin as mitotic regulator. Curr Opin Cell Biol 2006; 18: 616–622.
Yang YL, Li XM . The IAP family: endogenous caspase inhibitors with multiple biological activities. Cell Res 2000; 10: 169–177.
Eckelman BP, Salvesen GS . The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem 2006; 281: 3254–3260.
Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J 1998; 17: 2215–2223.
Li X, Yang Y, Ashwell JD . TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature 2002; 416: 345–347.
Zhao Y, Conze DB, Hanover JA, Ashwell JD . Tumor necrosis factor receptor 2 signaling induces selective c-IAP1-dependent ASK1 ubiquitination and terminates mitogen-activated protein kinase signaling. J Biol Chem 2007; 282: 7777–7782.
Tang ED, Wang CY, Xiong Y, Guan KL . A role for NF-kappaB essential modifier/IkappaB kinase-gamma (NEMO/IKKgamma) ubiquitination in the activation of the IkappaB kinase complex by tumor necrosis factor-alpha. J Biol Chem 2003; 278: 37297–37305.
Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin Jr AS . NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998; 281: 1680–1683.
Kuranaga E, Kanuka H, Tonoki A, Takemoto K, Tomioka T, Kobayashi M et al. Drosophila IKK-related kinase regulates nonapoptotic function of caspases via degradation of IAPs. Cell 2006; 126: 583–596.
Geisbrecht ER, Montell DJ . A role for Drosophila IAP1-mediated caspase inhibition in Rac-dependent cell migration. Cell 2004; 118: 111–125.
Leulier F, Lhocine N, Lemaitre B, Meier P . The Drosophila inhibitor of apoptosis protein DIAP2 functions in innate immunity and is essential to resist Gram-negative bacterial infection. Mol Cell Biol 2006; 26: 7821–7831.
Conze DB, Albert L, Ferrick DA, Goeddel DV, Yeh WC, Mak T et al. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol Cell Biol 2005; 25: 3348–3356.
Olayioye MA, Kaufmann H, Pakusch M, Vaux DL, Lindeman GJ, Visvader JE . XIAP-deficiency leads to delayed lobuloalveolar development in the mammary gland. Cell Death Differ 2005; 12: 87–90.
Ren J, Shi M, Liu R, Yang QH, Johnson T, Skarnes WC et al. The Birc6 (Bruce) gene regulates p53 and the mitochondrial pathway of apoptosis and is essential for mouse embryonic development. Proc Natl Acad Sci USA 2005; 102: 565–570.
Sanna MG, da Silva Correia J, Ducrey O, Lee J, Nomoto K, Schrantz N et al. IAP suppression of apoptosis involves distinct mechanisms: the TAK1/JNK1 signaling cascade and caspase inhibition. Mol Cell Biol 2002; 22: 1754–1766.
Plenchette S, Cathelin S, Rebe C, Launay S, Ladoire S, Sordet O et al. Translocation of the inhibitor of apoptosis protein c-IAP1 from the nucleus to the Golgi in hematopoietic cells undergoing differentiation: a nuclear export signal-mediated event. Blood 2004; 104: 2035–2043.
Whitesell L, Lindquist SL . HSP90 and the chaperoning of cancer. Nat Rev Cancer 2005; 5: 761–772.
Vischioni B, Giaccone G, Span SW, Kruyt FA, Rodriguez JA . Nuclear shuttling and TRAF2-mediated retention in the cytoplasm regulate the subcellular localization of cIAP1 and cIAP2. Exp Cell Res 2004; 298: 535–548.
Lewis J, Devin A, Miller A, Lin Y, Rodriguez Y, Neckers L et al. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J Biol Chem 2000; 275: 10519–10526.
Parcellier A, Brunet M, Schmitt E, Col E, Didelot C, Hammann A et al. HSP27 favors ubiquitination and proteasomal degradation of p27Kip1 and helps S-phase re-entry in stressed cells. FASEB J 2006; 20: 1179–1181.
Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD . Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 2000; 288: 874–877.
Zhao R, Houry WA . Molecular interaction network of the Hsp90 chaperone system. Adv Exp Med Biol 2007; 594: 27–36.
Voss AK, Thomas T, Gruss P . Mice lacking HSP90beta fail to develop a placental labyrinth. Development 2000; 127: 1–11.
Yue L, Karr TL, Nathan DF, Swift H, Srinivasan S, Lindquist S . Genetic analysis of viable Hsp90 alleles reveals a critical role in Drosophila spermatogenesis. Genetics 1999; 151: 1065–1079.
Inoue T, Hirata K, Kuwana Y, Fujita M, Miwa J, Roy R et al. Cell cycle control by daf-21/Hsp90 at the first meiotic prophase/metaphase boundary during oogenesis in Caenorhabditis elegans. Dev Growth Differ 2006; 48: 25–32.
Krone PH, Evans TG, Blechinger SR . Heat shock gene expression and function during zebrafish embryogenesis. Semin Cell Dev Biol 2003; 14: 267–274.
Didelot C, Lanneau D, Brunet M, Joly AL, De Thonel A, Chiosis G et al. Anti-cancer therapeutic approaches based on intracellular and extracellular heat shock proteins. Curr Med Chem 2007; 14: 2839–2847.
Kunisawa J, Shastri N . Hsp90alpha chaperones large C-terminally extended proteolytic intermediates in the MHC class I antigen processing pathway. Immunity 2006; 24: 523–534.
Yang QH, Du C . Smac/DIABLO selectively reduces the levels of c-IAP1 and c-IAP2 but not that of XIAP and livin in HeLa cells. J Biol Chem 2004; 279: 16963–16970.
Vaux DL, Silke J . IAPs, RINGs and ubiquitylation. Nat Rev Mol Cell Biol 2005; 6: 287–297.
Rebe C, Cathelin S, Launay S, Filomenko R, Prevotat L, L'Ollivier C et al. Caspase-8 prevents sustained activation of NF-kappaB in monocytes undergoing macrophagic differentiation. Blood 2007; 109: 1442–1450.
Ribeil JA, Zermati Y, Vandekerckhove J, Cathelin S, Kersual J, Dussiot M et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature 2007; 445: 102–105.
Yun BG, Matts RL . Differential effects of Hsp90 inhibition on protein kinases regulating signal transduction pathways required for myoblast differentiation. Exp Cell Res 2005; 307: 212–223.
Nimmanapalli R, O'Bryan E, Bhalla K . Geldanamycin and its analogue 17-allylamino-17-demethoxygeldanamycin lowers Bcr-Abl levels and induces apoptosis and differentiation of Bcr-Abl-positive human leukemic blasts. Cancer Res 2001; 61: 1799–1804.
Bae J, Mitsiades C, Tai YT, Bertheau R, Shammas M, Batchu RB et al. Phenotypic and functional effects of heat shock protein 90 inhibition on dendritic cell. J Immunol 2007; 178: 7730–7737.
Yu W, Rao Q, Wang M, Tian Z, Lin D, Liu X et al. The Hsp90 inhibitor 17-allylamide-17-demethoxygeldanamycin induces apoptosis and differentiation of Kasumi-1 harboring the Asn822Lys KIT mutation and downregulates KIT protein level. Leuk Res 2006; 30: 575–582.
Sordet O, Rebe C, Plenchette S, Zermati Y, Hermine O, Vainchenker W et al. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood 2002; 100: 4446–4453.
Pflieger D, Chabane S, Gaillard O, Bernard BA, Ducoroy P, Rossier J et al. Comparative proteomic analysis of extracellular matrix proteins secreted by two types of skin fibroblasts. Proteomics 2006; 6: 5868–5879.
Acknowledgements
We thank D Toft and D Picard for HSP90α- and β-specific antibodies, cDNA and useful discussions. We thank J Vinh (CNRS UMR 7637) for proteomic analysis and A Hammann for excellent technical assistance. We thank D Zatorska and H He for PU-H71 and PU-DZ8. This work was financially supported by ‘la Ligue Contre le Cancer’ (Nievre Committee), ‘l'Association pour la Recherche contre le Cancer’, ‘l'INSERM’ (to CG), the SynCure Cancer Research Foundation, The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center (MSKCC), H Goodwin, A Goodwin and the Commonwealth Cancer Foundation for Research (to CG). CD received a fellowship from the ‘Fondation pour la Recherche Medicale’ and ‘Fondation de France Leucémie’, AJ and SC from ‘La Ligue Nationale Contre le Cancer’ and DL from ‘Ministere de la Recherche et de l'Enseignement Supérieur’.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by S Kumar
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Rights and permissions
About this article
Cite this article
Didelot, C., Lanneau, D., Brunet, M. et al. Interaction of heat-shock protein 90β isoform (HSP90β) with cellular inhibitor of apoptosis 1 (c-IAP1) is required for cell differentiation. Cell Death Differ 15, 859–866 (2008). https://doi.org/10.1038/cdd.2008.5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2008.5
Keywords
This article is cited by
-
cIAP1/TRAF2 interplay promotes tumor growth through the activation of STAT3
Oncogene (2023)
-
Co-targeting HSP90 alpha and CDK7 overcomes resistance against HSP90 inhibitors in BCR-ABL1+ leukemia cells
Cell Death & Disease (2023)
-
Hsp90ab1 stabilizes LRP5 to promote epithelial–mesenchymal transition via activating of AKT and Wnt/β-catenin signaling pathways in gastric cancer progression
Oncogene (2019)
-
The HSP90 inhibitor, 17AAG, protects the intestinal stem cell niche and inhibits graft versus host disease development
Oncogene (2016)
-
Do not stress, just differentiate: role of stress proteins in hematopoiesis
Cell Death & Disease (2015)