Abstract
We have analyzed the incidence and risk factors for the occurrence of invasive aspergillosis (IA) among 219 consecutive recipients of an allogeneic hematopoietic SCT after a reduced-intensity conditioning regimen (Allo-RIC). Twenty-seven patients developed an IA at a median of 218 days (range 24–2051) post-Allo-RIC, for a 4-year incidence of 13% (95% confidence interval 4–24%). In multivariate analysis, risk factors for developing IA were steroid therapy for moderate-to-severe graft vs host disease (GVHD) (Hazard Ratio (HR) 2.9, P=0.03), occurrence of a lower respiratory tract infection (LRTI) by a respiratory virus (RV) (HR 4.3, P<0.01) and CMV disease (HR 2.8, P=0.03). Variables that decreased survival after Allo-RIC were advanced disease phase (HR 1.9, P=0.02), steroid therapy for moderate-to-severe GVHD (HR 2.2, P<0.01), not developing chronic GVHD (HR 4.3, P<0.01), occurrence of LRTI by an RV (HR 3.4, P<0.01) and CMV disease (HR 2, P=0.01), whereas occurrence of IA had no effect on survival (P=0.5). Our results show that IA is a common infectious complication after an Allo-RIC, which occurs late post-transplant and may not have a strong effect on survival. An important observation is the possible role of LRTI by conventional RVs as risk factors for IA.
Similar content being viewed by others
Introduction
Invasive fungal infections (IFIs) are a recognized cause of morbidity and mortality in patients who have undergone an allogeneic hematopoietic SCT (Allo-HSCT).1 The use of reduced-intensity conditioning (RIC) regimens may have changed the incidence and time distribution at which IFIs are seen after an Allo-HSCT.2, 3, 4 With regard to invasive aspergillosis (IA), which is by far the most common IFI at our institution and in many others, RIC regimens may have changed their epidemiology, but there are few studies on the description and risk factor analysis of IA in large patient cohorts with a long follow-up. Here, we report our findings regarding the incidence and risk factors for the development of IA among 219 adult patients who underwent an Allo-HSCT with an RIC (Allo-RIC) at the Hospital de La Santa Creu i Sant Pau, Barcelona, Catalonia (ES), between 1999 and 2007, and were prospectively monitored for the occurrence of IA. During this time period, symptomatic patients were also monitored for respiratory virus infections, allowing the analysis of any possible association of these with the occurrence of IA.
Patients and methods
Two hundred nineteen consecutive adults received an Allo-RIC based on fludarabine plus intermediate-dose BU or melphalan in our Division between 1999 and 2007. Detailed patient characteristics are shown in. Table 1. All patients were included in a series of consecutive Allo-RIC trials designed for patients not eligible for a conventional high-dose myeloablative conditioning, as reported elsewhere in detail.5, 6, 7 All patients gave written informed consent for inclusion in each study, and all studies were approved by our national and local Ethics Committees.
Conditioning regimen and graft-vs-host disease (GVHD) prophylaxis
In brief, two RIC regimens were used. Both included fludarabine plus either oral BU 8–10 mg/kg (with pharmakokinetic drug-dose adjustment8) or IV melphalan 70–140 mg/m2 (for myeloid and lymphoid malignancies, respectively).5 Alemtuzumab or antithymocyte globulin was given to recipients of HLA-mismatched related donors or volunteer unrelated donors (VUDs). Graft-vs-host disease (GVHD) prophylaxis consisted of CsA plus short-course MTX or CsA plus mycophenolate mofetil (MMF), as described elsewhere.6, 7, 8, 9
Antifungal prophylaxis
While hospitalized, patients at high risk for IA were kept in areas with a positive-pressure, high-efficiency particulate air-filtered isolation. Anti-Aspergillus antifungal prophylaxis was not routinely used, but patients with mucositis and risk of invasive candidiasis received fluconazole prophylaxis (200 mg/day). Thus, patients did not receive a prophylactic antifungal with activity against molds in the early, pre-engraftment period. After engraftment, if GVHD occurred that required high-dose steroids, patients again received antifungal prophylaxis, which consisted of fluconazole or itraconazole depending on the patient's tolerance and potential drug interactions. Patients who required hospitalization because of severe steroid-refractory GVHD received prophylactic therapy with a lipid formulation of amphotericin B at a dose of 1 mg/kg/ daily.10
Monitoring for aspergillosis and respiratory viral infections
Aspergillosis
The initial diagnostic work-up of febrile neutropenic patients and the use of empirical antibacterial treatment followed international guidelines.11 Follow-up included daily clinical examination and microbiological or radiological examinations as clinically indicated. Empirical antifungal therapy with anti-mold active agents was started in patients with persistent or recurrent fever of unknown origin (>72 h) and a thoracic computerized tomography (CT) scan was carried out, as reported earlier.12 Patients with GVHD and a febrile illness or respiratory symptoms not resolving with antibiotics were also routinely studied with a chest CT since 1995, and the routine study of the galactomannan (GM) Platelia assay (Bio-Rad Laboratories, Marres la Coquette, France) in blood samples since 2003, with a maximum of three times per week in inpatients and at each visit in outpatients. Respiratory samples were also always studied by fungal-directed cytological stains and fungal cultures. Any of these patients who developed a pulmonary infection of possible filamentous (mold) fungal origin is maintained in a prospective registry that is continuously updated by the senior author (RM).
Respiratory virus (RV) infections
Since 1998, all inpatient or outpatient adults with a hematologic malignancy (including HSCT recipients) who had signs and symptoms of an upper respiratory tract infection (URTI) or lower RTI (LRTI) underwent a detailed virological evaluation. Patients with symptoms of a URTI underwent nasopharyngeal aspiration (NPA), whereas patients with a LRTI underwent bronchoalveolar lavage (BAL) when it was clinically possible.13 Patients with pneumonia but no signs of URTI did not undergo NPA. All clinical samples were tested for viral Ag by indirect immunofluorescence assays for respiratory syncytial virus (RSV), parainfluenzae viruses1, 2, 3 (PIV−1, −2 or −3), influenza A or B (FluA or FluB) and adenoviruses (ADV). Samples were always cultured for these viruses (plus enterovirus) and tested by reverse-transcription PCR (RT-PCR) for non-polio enterovirus, as described elsewhere.14 In addition, aliquots from BAL and NPA samples were stored at −70 °C and tested retrospectively for metapneumoviruses and coronaviruses, as described in detail elsewhere.15
Definitions
Definition of IA
Proven and probable IA was defined by the 2002 European Organization for Research and Treatment of Cancer/Mycoses Study Group consensus guidelines.16 In brief, probable IA was defined by 2 or more positive results of serum galactomannan (optical index>0.5) or positive galactomannan in BAL fluid, or the isolation of Aspergillus sp. from a non-sterile site (BAL, one sample; sputum, two samples) or histologically-defined hyalohyphomycosis without a confirmatory culture, associated to suggestive clinical and radiological signs. Suggestive radiological findings included the presence of nodules with the halo sign during neutropenia or cavitated nodules after neutrophil recovery on the chest X-ray or CT scan.12
Respiratory virus (RV) infections
A URTI was defined as the new onset of nasal, pharyngeal or laryngeal irritation with isolation of an RV in an NPA. LRTI was defined as the new onset of cough, rales and/or wheezing in conjunction with a new pulmonary infiltrate in case of pneumonia identified on a chest radiograph or chest CT scan, with isolation of an RV in a BAL or lung sample.
CMV disease
This disease was divided into pneumonia, gastro intestinal disease and retinitis. CMV pneumonia was defined by the presence of signs and/or symptoms of pulmonary disease combined with the detection of CMV in BAL fluid or lung tissue samples. Detection of CMV was carried out by virus isolation, histopathologic testing, immunohistochemical analysis or in situ hybridization. The diagnosis of CMV gastro intestinal disease required a positive histology from the affected segment of the gastrointestinal tract, whereas CMV retinitis was defined by the characteristic retinal lesions identified by an expert ophthalmologist.17
Statistical analysis
The incidence of developing an IA was calculated from the time of transplantation using the cumulative incidence estimate, considering relapse or progression of the underlying malignancy and death from other causes as competing events, whereas patients who were still alive and progression-free at the time of reporting were censored at the last follow-up date.18, 19 The incidence of other post-transplant events, such as acute and chronic GVHD and infection by an RV was also calculated by cumulative incidence. OS was calculated with the Kaplan–Meier probability estimator from the time of transplantation to death from any cause, with censoring at last follow-up in survivors. Univariate analyses of the risk factors for developing IA were carried out using univariate Cox regression analysis, whereas the log-rank test was used for OS. In addition, to further characterize the truly relevant risk factors, multivariate testing was carried out by multivariate Cox regression analysis, including variables with a P<0.1 in univariate testing. The variables analyzed for their effect on the development of IA (and OS) are shown in Table 2 Post-transplant variables, such as the use of steroids for moderate-to-severe GVHD (defined as acute GVHD grades II-IV and/or extensive chronic GVHD), CMV infection, CMV disease, presence of monocytopenia (<0.1 × 109/l) and occurrence of a URTI or an LRTI by an RV were included as time-dependent covariates. The assumption of proportional hazards over time was tested for all explanatory covariates using a time-dependent covariate. All reported P values are two-sided, and a significance level of 0.05 was used. All statistical analyses were carried out using SPSS version 15.0 (SPSS, Chicago, IL, USA), with the exception of the cumulative incidence analyses, which were carried out with NCSS 2004 (no. Cruncher Statistical System, Kaysville, UT, USA). Patients were followed up to 31 August 2008.
Results
Twenty-seven patients developed a probable or proven IA post-Allo-RIC; two of these patients met the criteria for a possible IA before death, but the post-mortem examination confirmed the diagnosis. It is noted that there were no cases of non-Aspergillus invasive fungal infections. The 4-year cumulative incidence of IA was 13% (95% confidence interval (CI) 5–24%). The median day of onset of these infections was +218 post-Allo-RIC (range +24–+2051). Only nine cases (33%) started before day +100 post-transplant, whereas nine (33%) occurred from days +101–+365 and an additional nine cases after day +365. Aspergillus species were isolated from 18 cases, and consisted in 14 Aspergillus fumigatus, 3 A. flavus, 3 A. terreus and 2 Aspergillus niger. In 15 cases (56%) included since 2003, the galactomannan index in blood and/or BAL was positive, being the only (n=8) or an additional (n=7) microbiological criteria for the diagnosis of IA.
Risk factors for IA
The univariate and multivariate risk factors for IA are shown in Table 3 In the multivariate model, the only three significant variables associated with a higher risk of developing an IA were the use of steroid therapy for moderate-to-severe GVHD (Hazard Ratio (HR) 2.9, 95% 1.1–7.6, P=0.03), Figure 1 occurrence of CMV disease post-transplant (HR 2.8, 95% CI 1.1–6.8, P=0.03), Figure 2 and occurrence of an LRTI by an RV (HR 4.3, 95% CI 2–9.4, P<0.01) Figure 3 All of these variables were analyzed as time-dependent variables, and thus only those events that occurred before the onset of an IA were considered as risk factors for IA. Of the 19 patients in whom steroids were begun before the occurrence of IA, the median time interval between the start of steroid therapy and the onset of IA was 117 (range 1 to 552) days. Other variables such as duration of neutropenia and the variables shown in Table 2 did not significantly affect the risk for IA. With respect to the duration of neutropenia, the median day to reach a stable neutrophil count of 0.5 × 109/l was +14 (range, +7 to +23), occurring in 95% of cases between days +9 and +16; in addition, there were only nine delayed engraftments (beyond day +21) and no prolonged pancytopenia because of graft failure.
Four-year cumulative incidence of developing invasive pulmonary aspergillosis according to the need for steroid therapy of moderate-to-severe graft-versus-host disease. The P value refers to the multivariate Cox Regression analysis including the variable as a time-dependent covariate. The thin line refers to patients who received steroids and the dark line to those who did not receive steroids.
Four-year cumulative incidence of developing invasive pulmonary aspergillosis according to the development of CMV disease. The P value refers to the multivariate Cox Regression analysis including the variable as a time-dependent covariate. The thin line refers to patients who developed CMV disease and the dark line to those who did not develop CMV disease.
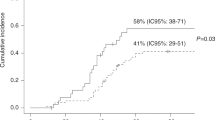
Four-year cumulative incidence of developing invasive pulmonary aspergillosis according to the development of lower respiratory tract infection by a conventional respiratory virus (LRTI-RV). The P value refers to the multivariate Cox Regression analysis including the variable as a time-dependent covariate. The thin line refers to patients who developed a LRTI-RV and the dark line to those who did not develop a LRTI-RV.
Types of RV isolated
During the study period, a total of 97 RVs were isolated from 77 patients, as 20 patients had at least two different RV infections diagnosed. The 4-year cumulative incidence of infection by a common RV was 32% (95% CI 24–40). The types of RVs isolated from patients with URTI and LRTI are shown in Table 3 Of the 27 patients who developed at least one episode of LRTI by an RV, 11 (41%) developed an IA after the RV infection, with a median interval of 22 days (range 1 to 61 days).
Effect of IA and RV infections on survival and non-relapse mortality
All patients received at least three days of one or more systemic therapies with anti-mold active antifungal agents (voriconazole in 11 cases, caspofungin in 10 cases and a lipid formulation of amphotericin B in 16 cases). The 4-month survival after the onset of IA was 48% (13/27 patients). We analyzed the 4-month survival according to the presence of five poor risk factors for survival recently published for allogeneic HSCT recipients with IA (monocytopenia (<0.1 × 109/l), disseminated aspergillosis, renal failure (creatinine>1.5 times the upper limit of normal, or ULN), elevated bilirubin (>2 times the ULN), respiratory failure (required supplemental oxygen to maintain a pO2>60 mmHg) and progression or advanced status of the underlying malignancy). The 4-month survival in patients with 0–1, 2–3 and 4–5 risk factors was 11/13 (85%), 2/9 (22%) and 0/5 (0%), respectively. The 4-year OS estimate for patients with an IA was 33%, compared with 53% for control patients (that is, those who did not develop IA) (P=0.2). In multivariate analysis, development of an IA (analyzed as a time-dependent post-transplant variable) did not have an effect on OS (P=0.5). Variables predictive of death were advanced disease phase (HR 1.9, (95% CI 1.4–2.6), (P=0.02)), steroid therapy for moderate-to-severe GVHD (HR 2.2, (95% CI 1.5–3.3), (P<0.01)), not developing chronic GVHD (HR 4.3, (95% CI 2.8–6.6), (P<0.01)), occurrence of LRTI by an RV (HR 3.4, (95% CI 1.8–6.3), (P<0.01)) and occurrence of CMV disease post-transplant (HR 2, (95% CI 1.2–3.5), (P=0.01)). We also analyzed the effect of developing an IA on non-relapse mortality (NRM). The 4-year cumulative incidence of NRM for the whole group was 27% (95% CI: 21–35). The factors associated with higher NRM in the multivariate analysis were: Acute GVHD grades II-IV (HR 3, (95% CI 1.5–6) (P=0.002)), poor performance status (ECOG>1) (HR 2.1, (95% CI 1.1–4) (P=0.02)), occurrence of CMV disease (HR 2 (95% CI 1–4.1) (P=0.05)), and occurrence of an LRTI by an RV (HR 2.4 (95% CI 1.1–5.3) (P=0.04)). The development of IA (analyzed as a time-dependent post-transplant variable) did not have an effect on NRM in univariate analysis (34% for patients with an IA compared with 24% for control patients (that is, those who did not develop IA) (P=0.3).
Discussion
This study represents one of the largest epidemiological studies on the occurrence of invasive mycoses after Allo-RIC. As only cases of IA occurred, without a single case of non-Aspergillus molds or invasive yeast infections, the study focuses on IA. In addition, the long follow-up and detailed prospective observation of all cases lends credibility to its results. The first surprise is the lack of IA in the early post-transplant period, a period during which the first group of neutropenia-related IA has been traditionally observed.20, 21 Possibly, the short duration of neutropenia and the low non-hematological toxicity are responsible for this early protection from IA, even without the use of the anti-Aspergillus drug prophylaxis. However, during longer follow-up, the risk of developing an IA was still substantial and very similar to the 15% incidence of IA traditionally observed after an Allo-HSCT with conventional myeloablative conditioning.3, 4, 22, 23, 24, 25, 26 In a review published in 2002, the median time to onset of IA after a total of nearly 7400 Allo-HSCT procedures was 90 days (range 19–115 days), 26 again emphasizing the late onset of the cases of IA in this study. When comparing the incidence of IA between studies, it is important to remember that in many studies the Kaplan–Meier P estimate method is used for analyzing the P of a post-transplant event (with at least one clear competing event). However, the recommended method is the cumulative incidence estimate, taking into account major competing events (in this case, relapse of the underlying disease is a competing event for the occurrence of an IA), as used in this study.18, 19 Nearly always, the incidence of a post-transplant event, such as the occurrence of an IA, will be significantly lower than the probability of that same event calculated by the typical Kaplan–Meier method.
Established risk factors for IA among Allo-HSCT recipients include duration of neutropenia before engraftment (for the early post-transplant period or in case of late graft failure), use and duration of glucocorticosteroids, donor type, underlying disease, age of transplant recipient, and presence and treatment of GVHD.3, 4, 22, 23, 24, 25, 26 This study is interesting because of several observations. As we closely monitored the occurrence of RV infections in all symptomatic patients, we were able to analyze their possible effect on the occurrence of IA. It has been known for a long time that apparently immunocompetent (or at least not severely immunocompromised) patients can develop an IA immediately after or during an RV lower respiratory tract infection. This association has been described mostly after influenza infections.20, 22, 27, 28, 29, 30, 31, 32 In addition, recent studies from two independent Allo-HSCT groups have reported that infection by an RV (influenza virus types A and B, parainfluenza, respiratory syncytial virus, adenovirus or other RVs) was an independent risk factor for developing IA in large retrospective analyses.20, 22, 27, 28, 29, 30, 31, 32 This study reinforces the role that RV infections may have in the development of IA after an Allo-HSCT. In addition, we found that only LRTIs increased the risk of developing an IA, whereas isolated URTIs had no effect. This association may reflect a direct implication of the alteration in the bronchial mucosa or pulmonary defensive mechanisms by the RV infection.33, 34 However, it could also simply reflect how the mechanisms or risk factors by which progression of an URTI to an LRTI by an RV occurs also predispose to the development of an IA.
Not unexpectedly, the occurrence of CMV disease and the need for steroid therapy to treat moderate-to-severe aGVHD and/or cGVHD were also identified by multivariate analysis as risk factors for IA, and other well-known risk factors had an effect in univariate analysis. These findings have important practical implications, as they highlight that in Allo-RIC recipients, strategies of anti-Aspergillus prophylaxis should be focused on patients who require steroids and/or develop clinically significant GVHD rather than on the neutropenic phase of the procedure in which IFIs are currently very infrequent. Another relevant consequence from our observations is that RIC regimens for allografting may carry a significant risk of developing late onset IA, and the use of prophylaxis may also be justified after CMV disease or after an LRTI by an RV. In addition, such prophylaxis should also be targeted to patients with other possible recently described risk factors for IA, such as those with hyperferritenemia/iron overload22 or those with donor/recipient genetic polymorphisms, which may predispose to this opportunistic infection.35 On the other hand, since the introduction of fluconazole prophylaxis, the risk of developing a severe invasive candidiasis post-transplant has decreased, and the current incidence and mortality because of yeast infections is well below 5% in most Allo-HSCT studies.26 This has been confirmed in this study.
Four-month mortality after the onset of IA is very high in Allo-HSCT recipients, usually in the range of 60–80%,1, 36, 37, 38 similar to the 52% mortality in the 27 patients included herein. Of interest is the fact that 4-month mortality differed markedly according to the no. of risk factors for 4-month survival. These variables were selected from those identified in three recent studies that analyzed the prognostic variables for survival in patients with an IA after an Allo-HSCT;1, 36, 37, 38 their usefulness in our small patient sample suggests that these results may be useful in clinical practice, although larger validation and prognostic model improvement studies are clearly needed.
It is unclear whether the high 4-month mortality after an IA is essentially attributable to the IA or may be a consequence of other underlying high-risk factors, which in turn increase the risk of developing such infections. It is thus interesting that in our multivariate survival analysis, we found that IA was not an independent risk factor for dying after Allo-RIC. However, owing to the limited ability of identifying all the truly independent risk factors for survival in such a complex clinical situation, we believe that this observation should be interpreted with caution, as most studies have shown that patients who develop an IA have a very poor 4–12 month survival.1, 3 However, recent results from the Seattle group showed that survival after an IA was improved in patients who had received a RIC regimen with respect to those who received a conventional myeloablative conditioning.1, 4 In addition, severe opportunistic infections are well known to coexist and possibly be involved in mortality in many such patients. Thus, it is probable that CMV disease and LRTI by an RV coexisted with IA at the time of death, making it difficult to interpret what the main cause of death is. On the other hand, the finding that LRTI by an RV did have a strong effect on OS lends support to the continued efforts to analyze the epidemiology and clinical effect of these infections in Allo-HSCT recipients, including virological studies in the routine post-transplant work-up of patients with URTIs and LRTIs.34
In conclusion, IA is an important cause of infectious morbidity after an Allo-RIC, although it occurs late post-transplant and may not have a strong effect on survival. An important observation is the possible role of LRTI by conventional RVs as risk factors for IA.
Conflict of interest
The authors declare no conflict of interest.
References
Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA . Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis 2007; 44: 531–540.
Martino R, Caballero MD, Canals C, San MJ, Sierra J, Rovira M et al. Reduced-intensity conditioning reduces the risk of severe infections after allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant 2001; 28: 341–347.
Barnes PD, Marr KA . Risks, diagnosis and outcomes of invasive fungal infections in haematopoietic stem cell transplant recipients. Br J Haematol 2007; 139: 519–531.
Fukuda T, Boeckh M, Carter RA, Sandmaier BM, Maris MB, Maloney DG et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood 2003; 102: 827–833.
Martino R, Caballero MD, Canals C, Simon JA, Solano C, Urbano-Ispizua A et al. Allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning: results of a prospective multicentre study. Br J Haematol 2001; 115: 653–659.
Perez-Simon JA, Martino R, Caballero D, Valcarcel D, Rebollo N, De la CR et al. Reduced-intensity conditioning allogeneic transplantation from unrelated donors: evaluation of mycophenolate mofetil plus cyclosporin A as graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant 2008; 14: 664–671.
Valcarcel D, Martino R, Caballero D, Martin J, Ferra C, Nieto JB et al. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: chronic graft-versus-host disease is the strongest factor improving survival. J Clin Oncol 2008; 26: 577–584.
Martino R, Perez-Simon JA, Moreno E, Queralto JM, Caballero D, Mateos M et al. Reduced-intensity conditioning allogeneic blood stem cell transplantation with fludarabine and oral busulfan with or without pharmacokinetically targeted busulfan dosing in patients with myeloid leukemia ineligible for conventional conditioning. Biol Blood Marrow Transplant 2005; 11: 437–447.
Perez-Simon JA, ez-Campelo M, Martino R, Brunet S, Urbano A, Caballero MD et al. Influence of the intensity of the conditioning regimen on the characteristics of acute and chronic graft-versus-host disease after allogeneic transplantation. Br J Haematol 2005; 130: 394–403.
Subira M, Martino R, Sureda A, Altes A, Briones J, Brunet S et al. Safety and efficacy of low-dose amphotericin B lipid complex for empirical antifungal therapy of neutropenic fever in patients with hematologic malignancies. Methods Find Exp Clin Pharmacol 2001; 23: 505–510.
Rolston KV . The Infectious Diseases Society of America 2002 guidelines for the use of antimicrobial agents in patients with cancer and neutropenia: salient features and comments. Clin Infect Dis 2004; 39 (Suppl 1) 1: S44–S48.
Hidalgo A, Parody R, Martino R, Sanchez F, Franquet T, Gimenez A et al. Correlation between high-resolution computed tomography and galactomannan antigenemia in adult hematologic patients at risk for invasive aspergillosis. Eur J Radiol (e-pub ahead of print 9 May 2008).
Martino R, Porras RP, Rabella N, Williams JV, Ramila E, Margall N et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant 2005; 11: 781–796.
Parody R, Rabella N, Martino R, Otegui M, del CM, Coll P et al. Upper and lower respiratory tract infections by human enterovirus and rhinovirus in adult patients with hematological malignancies. Am J Hematol 2007; 82: 807–811.
Williams JV, Martino R, Rabella N, Otegui M, Parody R, Heck JM et al. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J Infect Dis 2005; 192: 1061–1065.
Ascioglu S, Rex JH, De PB, Bennett JE, Bille J, Crokaert F et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002; 34: 7–14.
Ljungman P, Griffiths P, Paya C . Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34: 1094–1097.
Klein JP, Rizzo JD, Zhang MJ, Keiding N . Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part 2: Regression modeling. Bone Marrow Transplant 2001; 28: 1001–1011.
Klein JP, Rizzo JD, Zhang MJ, Keiding N . Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part I: unadjusted analysis. Bone Marrow Transplant 2001; 28: 909–915.
Marr KA, Carter RA, Boeckh M, Martin P, Corey L . Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 2002; 100: 4358–4366.
Junghanss C, Marr KA, Carter RA, Sandmaier BM, Maris MB, Maloney DG et al. Incidence and outcome of bacterial and fungal infections following nonmyeloablative compared with myeloablative allogeneic hematopoietic stem cell transplantation: a matched control study. Biol Blood Marrow Transplant 2002; 8: 512–520.
Garcia-Vidal C, Upton A, Kirby KA, Marr KA . Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis 2008; 47: 1041–1050.
Jantunen E, Nihtinen A, Anttila VJ . Changing landscape of invasive aspergillosis in allogeneic stem cell transplant recipients. Transpl Infect Dis 2008; 10: 156–161.
Mihu CN, King E, Yossepovitch O, Taur Y, Jakubowski A, Pamer E et al. Risk factors and attributable mortality of late aspergillosis after T-cell depleted hematopoietic stem cell transplantation. Transpl Infect Dis 2008; 10: 162–167.
Martino R, Subira M . Invasive fungal infections in hematology: new trends. Ann Hematol 2002; 81: 233–243.
Martino R, Subira M, Rovira M, Solano C, Vazquez L, Sanz GF et al. Invasive fungal infections after allogeneic peripheral blood stem cell transplantation: incidence and risk factors in 395 patients. Br J Haematol 2002; 116: 475–482.
Hasejima N, Yamato K, Takezawa S, Kobayashi H, Kadoyama C . Invasive pulmonary aspergillosis associated with influenza B. Respirology 2005; 10: 116–119.
Vandenbos F, Mondain-Miton V, Roger PM, Saint-Paul MC, Dellamonica P . Invasive pulmonary aspergillosis during influenza: a fortuitous association?]. Presse Med 1999; 28: 1755.
Alba D, Gomez-Cerezo J, Cobo J, Ripoll MM, Molina F, Vazquez JJ . Invasive pulmonary aspergillosis associated with influenza virus]. An Med Interna 1996; 13: 34–36.
Shapiro D, Ferriss J . Influenza A and aspergillosis. Chest 1986; 89: 318–319.
Lewis M, Kallenbach J, Ruff P, Zaltzman M, Abramowitz J, Zwi S . Invasive pulmonary aspergillosis complicating influenza A pneumonia in a previously healthy patient. Chest 1985; 87: 691–693.
Mezger M, Steffens M, Beyer M, Manger C, Eberle J, Toliat MR et al. Polymorphisms in the chemokine (C-X-C motif) ligand 10 are associated with invasive aspergillosis after allogeneic stem-cell transplantation and influence CXCL10 expression in monocyte-derived dendritic cells. Blood 2008; 111: 534–536.
Alonso JM . Immunity and pathophysiology of respiratory tract infections. Med Mal Infect 2008; 38: 433–437.
Boeckh M . The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol 2008; 143: 455–467.
Bochud PY, Chien JW, Marr KA, Leisenring WM, Upton A, Janer M et al. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N Engl J Med 2008; 359: 1766–1777.
Cordonnier C, Ribaud P, Herbrecht R, Milpied N, Valteau-Couanet D, Morgan C et al. Prognostic factors for death due to invasive aspergillosis after hematopoietic stem cell transplantation: a 1-year retrospective study of consecutive patients at French transplantation centers. Clin Infect Dis 2006; 42: 955–963.
Subira M, Martino R, Franquet T, Puzo C, Altes A, Sureda A et al. Invasive pulmonary aspergillosis in patients with hematologic malignancies: survival and prognostic factors. Haematologica 2002; 87: 528–534.
Nivoix Y, Velten M, Letscher-Bru V, Moghaddam A, Natarajan-Ame S, Fohrer C et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis 2008; 47: 1176–1184.
Acknowledgements
JL Piñana is supported by grants from the Instituto de Salud Carlos III (Expediente CM06/00139, Ministerio de Sanidad, Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martino, R., Piñana, J., Parody, R. et al. Lower respiratory tract respiratory virus infections increase the risk of invasive aspergillosis after a reduced-intensity allogeneic hematopoietic SCT. Bone Marrow Transplant 44, 749–756 (2009). https://doi.org/10.1038/bmt.2009.78
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2009.78
Keywords
This article is cited by
-
Low Rate of Invasive Fungal Infections During Induction and Consolidation Chemotherapy for Adults with De Novo Acute Myeloid Leukemia Without Anti-mold Prophylaxis: Single-Center 2002–2018 Empirical/Pre-emptive Approach
Mycopathologia (2020)
-
Foiling fungal disease post hematopoietic cell transplant: review of prophylactic strategies
Bone Marrow Transplantation (2018)
-
Validation of a new integrated prognostic score to predict non-relapse mortality in patients undergoing reduced-intensity conditioning allogeneic hematopoietic cell transplantation
Bone Marrow Transplantation (2015)
-
Incidence, characteristics and risk factors of marked hyperbilirubinemia after allogeneic hematopoietic cell transplantation with reduced-intensity conditioning
Bone Marrow Transplantation (2012)
-
Black hole in the lung
Bone Marrow Transplantation (2011)