Abstract
Phoenixin (PNX) is a newly discovered peptide that has been linked to reproductive function, both in the hypothalamus and pituitary. This review will focus on the most recent discoveries related to this novel neuropeptide. Initially, it was found that PNX increased gonadotropin releasing hormone (GnRH)-stimulated luteinizing hormone (LH) release from pituitary cells. Importantly, knockdown of PNX in female rats extended the estrous cycle by 2.3 days. Using novel hypothalamic cell lines, we found that PNX has a stimulatory role on kisspeptin (Kiss) and GnRH gene expression and secretion. The PNX receptor was uncovered using siRNA knockdown of GPR173, an orphan receptor postulated to bind PNX. We have found that the PNX-R signaling through protein kinase A (PKA) in hypothalamic neurons. Althuogh a number of studies demonstrate that PNX plays an important role in reproductive function, there is also evidence that it may have other functions, regulating the heart, feeding, memory, and anxiety, both in the brain and the periphery.
Similar content being viewed by others
Introduction
Phoenixin (PNX), first identified in 2013 by Yosten et al, is a highly conserved, secreted peptide1. The two main isoforms of PNX are 14 (PNX-14) and 20 (PNX-20) amino acids long1. Initial characterization of PNX found that it was crucial for normal reproductive function1. In the four years since, the involvement of PNX in reproduction has become more established and its receptor has been identified. Further, there is some evidence that PNX may have some effects on the heart, feeding, memory, and anxiety2,3,4,5,6,7,8. This review will provide further evidence for the role of PNX in reproductive function, as well as recent literature that suggests PNX is also involved in other facets of brain-mediated and peripheral physiology.
SMIM20 gene and PNX
PNX is cleaved from the C-terminal of small integral membrane protein 20 (SMIM20). SMIM20 contains proteolytic basic sites that coincide with the PNX-14 and PNX-20 sequences, thus PNX is predicted to be cleaved by a prohormone convertase and secreted1,9. However, this has not been experimentally confirmed and it is possible that PNX is cleaved by ectodomain shedding9, a regulated process whereby membrane proteins are cleaved10.
Little is known about SMIM20 itself, but it is conserved in humans, cows, rodents and zebrafish11. The only identified role of SMIM20 is in the mitochondria, where it acts as a chaperone-like protein11. It forms part of the mitochondrial translation regulation assembly intermediate of cytochrome c oxidase (MITRAC) and stabilizes a subunit of cytochrome c oxidase (COX), an essential part of the electron transport chain11. If levels of SMIM20 are too high or too low, COX cannot assemble11. An investigation into what can alter SMIM20 expression would also have implications for PNX expression.
PNX expression throughout the body
Besides their length, the only observed difference between PNX-14 and PNX-20 is that they are expressed at variable levels in specific tissues. PNX-20 is the predominant isoform in the hypothalamus1, while PNX-14 is predominant in the heart and spinal cord1,12. The few studies that have tested both PNX-14 and PNX-20 have observed no differences in their effects1,12. However, the isoforms must be amidated at the C-terminal as it has been shown that the non-amidated form is not biologically active13.
Using an enzyme-linked immunoassay targeted to the amidated end of PNX, Yosten et al showed that in rats, the hypothalamus is the area with the greatest expression of PNX with 2851 pg/g of tissue1. Using mass spectrometry, PNX-20 was shown to be the predominant isoform. When regional expression was investigated using immunohistochemistry with an antibody targeted to the amidated C-terminal end of PNX, highest expression was detected in the paraventricular and supraoptic nuclei1. PNX was also detected in numerous other hypothalamic regions including the ventromedial hypothalamus, arcuate (Arc) nucleus, lateral hypothalamus and dorsal hypothalamus, where it is partially co-expressed with nesfatin-1, another peptide with many different roles1,14. Elsewhere in the brain, PNX was identified in the nucleus of the solitary tract, the substantia nigra reticulata, dorsal motor nucleus of the vagus, area postrema and in the spinal cord1,12,13,15. PNX was also found in the anterior and posterior pituitary, as well as in the median eminence1. A recent study by Prinz et al examined expression of PNX-14 in rats and observed expression in many of the above-mentioned areas, but also identified PNX in the amygdala and spinocerebellar tract15.
The second highest expression of PNX was detected in the heart with approximately 500 pg/g of tissue1,4. Yosten et al also showed PNX expression in the stomach, esophagus, spleen, kidney and lungs1, however this was not replicated by Prinz et al15. PNX-14 was detected in the crypts of the duodenum, jejunum and ileum, as well as in the endocrine pancreas1,15.
Expression of PNX in human tissues has not been studied but PNX has been detected in serum at an average concentration of 0.7 ng/mL in obese men and 0.289±0.046 ng/mL in normal weight women8,16. The differences in concentrations may be due to the effects of weight or sex on PNX levels, however this has not been studied in detail.
Central effects of PNX
Reproduction
The first paper on PNX associated it with the hypothalamic-pituitary-gonadal (HPG) axis1, which coordinates reproductive function. Yosten et al observed PNX immunoreactivity in the hypothalamus and median eminence, and thus hypothesized that PNX could act on the anterior pituitary. While PNX treatment alone was insufficient to alter hormone release in primary pituitary culture, pretreatment with either PNX-14 or PNX-20 increased gonadotropin releasing hormone (GnRH)-stimulated luteinizing hormone (LH) release. Furthermore, this increase was mediated by a PNX-stimulated upregulation of the GnRH receptor (GnRH-R). A more recent paper by the same group demonstrated that intracerebroventricular (ICV) administration of PNX-20 in diestrous rats significantly increased LH plasma concentration 5 and 10 min later3. Functionally, siRNA knockdown of PNX in female rats extended the estrous cycle by 2.3 days or 58%1. These first results established the involvement of PNX in reproduction.
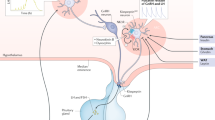
PNX is most highly expressed in the hypothalamus, an area critical for reproductive function1. The hypothalamus, however, is highly heterogeneous, making it difficult to study functions of specific neuronal populations17. Therefore, to investigate the effects of PNX on reproductive neurons of the hypothalamus, Treen et al used immortalized hypothalamic neurons representing GnRH- and Kiss-expressing neuronal populations (2 and Figure 1). As described previously, the mHypoA-GnRH/GFP cell line was derived from the hypothalamus of a mouse expressing GFP under control of the GnRH promoter18. The cells were immortalized with SV40 T-antigen and GFP-expressing cells were collected using fluorescence activated cell sorting. The Kiss cell lines, the mHypoA-Kiss/GFP-3 and mHypoA-Kiss/GFP-4, were generated in a similar manner, and represent Arc nucleus- or anteroventral periventricular (AVPV)-derived Kiss-expressing neurons, respectively19. The Arc population regulates pulsatile secretion of GnRH and the AVPV population regulates the preovulatory surge20. Treatment with 1000 nmol/L PNX-20 for 1 h increased GnRH secretion in the mHypoA-GnRH/GFP line and treatment with 10 and 100 nmol/L increased GnRH mRNA levels at 2 and 8 h, respectively2. GnRH-R mRNA was also increased with 100 nmol/L PNX at 2 and 8 h. In support of these findings, injection of PNX-14 into the anterior hypothalamic area increases GnRH expression and plasma concentration after 15 min7. In the mHypoA-Kiss/GFP-3 cell line, Kiss1 mRNA expression was upregulated at 24 h with 100 nmol/L PNX-202. Together, this indicates that PNX is a positive regulator of the HPG axis. Treen et al also demonstrated that the G-protein-coupled receptor, GPR173, mediates the effects of PNX in the hypothalamus2. The receptor for PNX was postulated to be GPR173 based on a ligand-binding assay conducted by Stein et al3. Treen et al demonstrated that GPR173 was expressed in the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell lines, and functional analysis through knockdown of GPR173 with siRNA abolished the PNX-mediated induction of GnRH, GnRH-R and Kiss12. GPR173 was found to be a Gαs-coupled receptor as PNX exposure increased pCREB, while inhibiting PKA blocked the effect of PNX on GnRH and Kiss1. Interestingly, PNX also regulated transcription factors Oct-1 and C/EBP-beta, both previously shown to be necessary for the regulation of GnRH gene expression. Further study on the role of GPR173 in the hypothalamus has shown that PNX is crucial for normal estrous cycling3. ICV injection of siRNA targeted to GPR173 doubled the length of the estrous cycle in female rats and eliminated the PNX-induced increase in plasma LH in diestrous rats3. These studies indicate that in rodents, GPR173 is a receptor for PNX.
Summary of the proposed mechanisms involved in the regulation of gene expression by PNX in mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell models. GPR173 has been identified as the cognate receptor for GPR173 (PNX-R). PNX increases the phosphorylation of CREB, suggesting that it activates a Gαs protein and the cAMP/PKA pathway. PKA was shown to be necessary to induce changes in GnRH, Kiss-1, Oct-1 and C/EBPb mRNA expression by PNX. This pathway may therefore mediate the hypothalamic changes in mRNA expression by PNX (from Treen et al2).
In humans, a study on PNX and reproduction has been published that investigated serum concentrations of PNX-14 in women with polycystic ovary syndrome (PCOS)16. PCOS is characterized by elevated androgen levels, polycystic ovaries and metabolic dysfunction21. Compared to control women, PNX-14 was elevated in patients with PCOS16. Since PCOS is also characterized by increased GnRH pulses21, it is conceivable that this is partially mediated by elevated PNX levels. Indeed, PNX-14 levels in PCOS patients were positively correlated with LH and total testosterone, which are downstream of GnRH in the HPG axis16. PNX-14 was also correlated positively with BMI, potentially implicating it in metabolic disorders.
Feeding behaviour
Central control of feeding behaviour plays an essential role in metabolic homeostasis. In addition to correlations between PNX and BMI, PNX has been shown to modulate food intake and feeding behaviour4,5. Rats are active in the dark phase and are inactive in the light phase. ICV injection of PNX-14 in the light phase, but not in the dark phase, increased food intake in the light phase5. It increased meal size and decreased meal interval, suggesting a reduction in satiation and satiety, respectively5. Since rats are active in the dark phase, it was hypothesized that due to the higher presence of orexigenic signals in this period, exogenous PNX-14 was not enough to cause a further increase in feeding5. The mechanism of action of PNX on feeding is primarily central, as intraperitoneal (IP) injection of PNX-14 during the light phase had no effect on feeding5. If PNX has an orexigenic role, it would be expected to be elevated prior to feeding; however Rocca et al found that post-prandial serum levels of PNX-14 were increased compared to pre-prandial levels, suggesting a potential anorexigenic role4. Perhaps this discrepancy could be due to the concentration of PNX administered, which could possibly have different effects at specific doses. It is unknown whether PNX displays circadian rhythmicity and because it was measured at the same time of day following 3 h of feeding, the increase following a meal could be a coincidence. Interestingly, the post-prandial increase in PNX was abolished in diet-induced obese rats4. Obesity is associated with insulin and leptin resistance, states in which cells are less responsive to these hormones, ultimately disrupting energy balance22. Therefore, pathways that undergo resistance in an obese state may regulate PNX.
Nutrients, such as sugars and fatty acids, affect reproductive signaling23,24. The fatty acids, palmitate and docosahexaenoic acid, increases GnRH expression and GnRH neurons have been shown to directly sense glucose in the blood23,24. Further research is necessary to understand the role of PNX in altering food intake, but also how PNX itself is affected in response to nutrient status.
Memory and anxiety
Behavioural studies in animals established the effects of PNX on enhancing memory and reducing anxiety. In a novel object recognition test, 25 nmol PNX-14 injection improved memory retention 3 d after a 10 s training period6. Similar results were obtained with an object location recognition test. Injection of PNX-14 or PNX-20 in mice extended the amount of time they spent in the centre of an open field test and the open arms of an elevated plus maze, indicating a reduction in anxiety7. Interestingly, when combined with a GnRH receptor antagonist, cetrorelix, there was no improvement in memory retention or anxiety, indicating that this effect was mediated through a GnRH-mediated pathway6,7. Additional experiments in disease states corroborated these findings. For instance, authors showed that PNX-14 injected into the lateral ventricle reversed the memory impairment induced by Aβ1-42 and scopolamine, a model of dementia6. Furthermore, a study in obese men showed that there was a negative correlation between PNX plasma concentration and anxiety8. Therefore, PNX and its signalling pathways may have potential therapeutic applications against memory impairment and anxiety.
Beyond the brain
Heart
Next to the hypothalamus, PNX is most highly expressed in the heart1,4. Perfusion of rat hearts with 100 pmol/L to 100 nmol/L PNX-14 decreased contractility and relaxation, therefore reducing stress on the heart4. During ischemia, the myocardium is damaged due to coronary artery blockage; however reperfusion, while necessary, also leads to injury25. Post-conditioning agents injected following infarction, such as tumor necrosis factor alpha or glucagon-like-peptide 2, may mitigate damage25,26. Injection of 0.5 nmol/L PNX into the heart following ischemia improved systolic and diastolic function as measured by dLVP and LEVDP, respectively4. PNX-14 reduced the infarct size and decreased myocardial apoptosis by blocking upregulation of pro-apoptotic genes, such as Bax and caspase 3, and increasing expression of the anti-apoptotic gene, Bcl-2. PNX was proposed to be mediating these effects through the reperfusion injury salvage kinase (RISK) and survival activating factor enhancement (SAFE) pathways. RISK activates pro-survival kinases that acutely protect the heart by inhibiting apoptosis, while SAFE acts through STAT327,28. Reduction in phosphorylation of components of these pathways after ischemia and reperfusion was reversed upon PNX administration4. Furthermore, inhibition of components of the RISK and SAFE pathways, including PI3K, NOS, MAPKK1 and mitochondrial potassium ATP channels, blocked the beneficial effects of PNX. It has yet to be studied whether endogenous levels of PNX could benefit cardiac function.
Spinal cord
In the spinal cord, PNX-14 is the predominant form of PNX, where it is involved in nociception and is pruritogenic12,13. Intrathecal injection of PNX decreased writhing after an IP acetic acid test, while injection of PNX antiserum increased writhing13. In contrast, tail flick latency in response to focused light was not significantly altered after intrathecal PNX injection13. Therefore, PNX can reduce visceral pain but not thermal pain13. In another paper, the authors noted that PNX had a similar distribution in the dorsal horn and dorsal root ganglion as gastrin-releasing peptide, which causes itching12. PNX was identified in the epidermis and dermis, and fluorogold injection revealed that PNX-expressing dorsal root ganglion cell bodies project to the skin. PNX-14 and PNX-20 injection to the back of the neck increased scratching, which was determined to be through a known itch-inducing receptor, the kappa opioid receptor. Whether PNX is involved in other sensory modalities has not been studied, nor has its function been investigated in other peripheral areas.
Receptor and signaling
Currently, PNX is known to bind and signal through GPR173, but this may not be its only receptor. GPR173 was identified using a ligand-binding assay, and was shown to be crucial for the effects of PNX on GnRH, Kiss1 and normal estrous cycling2,3. Previously, GPR173 was only known to bind the GnRH metabolite, GnRH-(1-5)29. Similar to PNX, GPR173 is highly conserved across species30. In humans, GPR173 is expressed in the hypothalamus, pituitary and ovaries, coinciding with the HPG axis31. It has been found at lower levels in peripheral tissues where PNX has been studied, such as in the heart and skin31. However, it has also been detected in tissues where PNX has not been studied, such as in the spleen and adrenal glands, suggesting that PNX may have specific functions in these tissues31. GPR173 has been found to couple a Gαs protein and activate PKA, but it has also been hypothesized to bind a Gq/112,32. It has been proposed that PNX may act through other receptors and signaling pathways. For example, PNX improves recovery after a myocardial infarction through the RISK and SAFE pathways, which involve signaling proteins other than those downstream of Gαs4. Additionally, the pruritogenic effect of PNX was mediated through the kappa opioid receptor, but it is unknown whether this was a direct or indirect mechanism12. To advance the characterization of PNX, further studies on the PNX receptor and signaling pathways will be critical.
GnRH and PNX
GnRH mediates many of the functions of PNX described in the literature. PNX increases GnRH and GnRH-R mRNA in immortalized GnRH-expressing neurons and increases GnRH-R expression in pituitary culture1,2. PNX is thought to be involved in the preovulatory LH surge through stimulation of GnRH1. Given that GnRH also stimulates puberty33, PNX may also be involved in its initiation. The PNX-mediated induction of GnRH and its receptor appears to have impacts beyond reproduction as a GnRH-R antagonist blocks the effects of PNX on anxiety and memory6,7. The anti-inflammatory functions of GnRH may also implicate PNX as an anti-inflammatory compound (34 and Wellhauser and Belsham, unpublished data). This could suggest a therapeutic role for PNX in inflammatory diseases, such as obesity. In obesity, PNX levels are disrupted as it has been observed that post-prandial PNX levels failed to increase in obese rats4, while mice on a high fat diet have elevated hypothalamic expression of the PNX gene (Wellhauser and Belsham, unpublished data). Further investigation is needed to determine whether PNX has anti-inflammatory effects, either on its own or through potentiation of GnRH signaling.
Conclusion
In summary, the recently identified peptide, PNX, has roles in a number of processes. PNX positively regulates HPG axis signaling, reduces cardiac reperfusion injury, modulates feeding, improves memory and decreases pain and anxiety. Due to its widespread expression throughout the body, PNX likely has many functions that have yet to be discovered. To further elucidate its effects, tissue-specific knockdown of PNX should be conducted. In addition, determining what regulates the expression of PNX itself could help identify its physiological role. Further functional characterization of PNX, through transgenic and whole genome analysis, will lead to a deeper understanding of biological processes.
References
Yosten GL, Lyu RM, Hsueh AJ, Avsian-Kretchmer O, Chang JK, Tullock CW, et al. A novel reproductive peptide, phoenixin. J Neuroendocrinol 2013; 25: 206–5.
Treen AK, Luo V, Belsham DD . Phoenixin activates immortalized GnRH and Kisspeptin neurons through the novel receptor GPR173. Mol Endocrinol 2016; 30: 872–88.
Stein LM, Tullock CW, Mathews SK, Garcia-Galiano D, Elias CF, Samson WK, et al. Hypothalamic action of phoenixin to control reproductive hormone secretion in females: importance of the orphan G protein-coupled receptor Gpr173. Am J Physiol 2016; 31: 489–96.
Rocca C, Scavello F, Granieri MC, Pasqua T, Amodio N, Imbrogno S, et al. Phoenixin-14: detection and novel physiological implications in cardiac modulation and cardioprotection. Cell Mol Life Sci 2018; 75: 743–56.
Schalla M, Prinz P, Friedrich T, Scharner S, Kobelt P, Goebel-Stengel M, et al. Phoenixin-14 injected intracerebroventricularly but not intraperitoneally stimulates food intake in rats. Peptides 2017; 96: 53–60.
Jiang JH, He Z, Peng YL, Jin WD, Wang Z, Mu LY, et al. Phoenixin-14 enhances memory and mitigates memory impairment induced by Aβ1-42 and scopolamine in mice. Brain Res 2015; 1629: 298–308.
Jiang JH, He Z, Peng YL, Jin WD, Mu J, Xue HX, et al. Effects of Phoenixin-14 on anxiolytic-like behavior in mice. Behav Brain Res 2015; 286: 39–48.
Hofmann T, Weibert E, Ahnis A, Elbelt U, Rose M, Klapp BF, et al. Phoenixin is negatively associated with anxiety in obese men. Peptides 2017; 88: 32–6.
Treen AK . The effects of the novel reproductive peptide phoenixin-20 amide on GnRH and kisspeptin hypothalamic cell models. Mol Endocrinol 2016; 30: 872–88.
Hayashida K, Bartlett AH, Chen Y, Park PW . Molecular and cellular mechanisms of ectodomain shedding. Anat Rec (Hoboken) 2010; 293: 925–37.
Dennerlein S, Oeljeklaus S, Jans D, Hellwig C, Bareth B, Jakobs S, et al. MITRAC7 acts as a COX1-specific chaperone and reveals a checkpoint during cytochrome c oxidase assembly. Cell Rep 2015; 12: 1644–55.
Cowan A, Lyu RM, Chen YH, Dun SL, Chang JK, Dun NJ . Phoenixin: A candidate pruritogen in the mouse. Neuroscience 2015; 310: 541–8.
Lyu RM, Huang XF, Zhang Y, Dun SL, Luo JJ, Chang JK, et al. Phoenixin: a novel peptide in rodent sensory ganglia. Neuroscience 2013; 250: 622–31.
Pałasz A, Rojczyk E, Bogus K, Worthington JJ, Wiaderkiewicz R . The novel neuropeptide phoenixin is highly co-expressed with nesfatin-1 in the rat hypothalamus, an immunohistochemical study. Neurosci Lett 2015; 592: 17–21.
Prinz P, Scharner S, Friedrich T, Schalla M, Goebel-Stengel M, Rose M, et al. Central and peripheral expression sites of phoenixin-14 immunoreactivity in rats. Biochem Biophys Res Commun 2017; 493: 195–201.
Ullah K, ur Rahman T, Wu DD, Lin XH, Liu Y, Guo XY, et al. Phoenixin-14 concentrations are increased in association with luteinizing hormone and nesfatin-1 concentrations in women with polycystic ovary syndrome. Clin Chim Acta 2017; 471: 243–7.
Wellhauser L, Gojska NM, Belsham DD . Delineating the regulation of energy homeostasis using hypothalamic cell models. Front Neuroendocrinol 2015; 36: 130–49.
McFadden SA, Menchella JA, Chalmers JA, Centeno ML, Belsham DD . Glucose responsiveness in a novel adult-derived GnRH cell line, mHypoA-GnRH/GFP: involvement of AMP-activated protein kinase. Mol Cell Endocrinol 2013; 377: 65–74.
Treen AK, Luo V, Chalmers JA, Dalvi PS, Tran D, Ye W, et al. Divergent regulation of ER and Kiss genes by 17β-estradiol in hypothalamic ARC versus AVPV models. Mol Endocrinol 2016; 30: 217–33.
Gottsch ML, Clifton DK, Steiner RA . Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive axis. Mol Cell Endocrinol 2006; 254-255: 91–6.
Azziz R, Carmina E, Chen Z, Dunaif A, Laven JSE, Legro RS, et al. Polycystic ovary syndrome. Nat Rev Dis Primers 2016; 2: 16057.
Kahn BB, Flier JS . Obesity and insulin resistance. J Clin Invest 2000; 106: 473–81.
Tran DQ, Ramos EH, Belsham DD . Induction of Gnrh mRNA expression by the omega-3 polyunsaturated fatty acid docosahexaenoic acid and the saturated fatty acid palmitate in a GnRH-synthesizing neuronal cell model, mHypoA-GnRH/GFP. Mol Cell Endocrinol 2016; 426: 125–35.
Roland AV, Moenter SM . Regulation of gonadotropin-releasing hormone neurons by glucose. Trends Endocrinol Metab 2011; 22: 443–9.
Lacerda L, Somers S, Opie LH, Lecour S . Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res 2009; 84: 201–8.
Penna C, Pasqua T, Perrelli MG, Pagliaro P, Cerra MC, Angelone T . Postconditioning with glucagon like peptide-2 reduces ischemia/reperfusion injury in isolated rat hearts: role of survival kinases and mitochondrial KATP channels. Basic Res Cardiol 2012; 107: 272. doi: 10.1007/s00395-012-0272-6.
Hausenloy DJ, Yellon DM . Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Failure Rev 2007; 12: 217–34.
Lecour S . Activation of the protective survivor activating factor enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? J Mol Cell Cardiol 2009; 47: 32–40.
Larco DO, Cho-Clark M, Mani SK, Wu TJ . The metabolite GnRH-(1-5) inhibits the migration of immortalized GnRH neurons. Endocrinology 2013; 154: 783–95.
Matsumoto M, Saito T, Takasaki J, Kamohara M, Sugimoto T, Kobayashi M, et al. An evolutionarily conserved G-protein coupled receptor family, SREB, expressed in the central nervous system. Biochem Biophys Res Commun 2000; 272: 576–82.
Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics 2014; 13: 397–406.
Larco DO, Semsarzadeh NN, Cho-Clark M, Mani SK, Wu TJ . β-Arrestin 2 is a mediator of GnRH-(1–5) signaling in immortalized GnRH neurons. Endocrinology 2013; 154: 4726–36.
Plant TM . Neuroendocrine control of the onset of puberty. Front Neuroendocrinol 2015; 38: 73–88.
Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, et al. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 2013; 497: 211–6.
Acknowledgements
We acknowledge funding from the Canadian Institutes for Health Research (CIHR) and Canada Foundation for Innovation and Canada Research Chairs Program (DDB). EKM was supported by a Natural Sciences and Engineering Research Council (NSERC) Studentship.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Mcilwraith, E., Belsham, D. Phoenixin: uncovering its receptor, signaling and functions. Acta Pharmacol Sin 39, 774–778 (2018). https://doi.org/10.1038/aps.2018.13
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2018.13
Keywords
This article is cited by
-
G protein-coupled receptors in neurodegenerative diseases and psychiatric disorders
Signal Transduction and Targeted Therapy (2023)
-
Phoenixin-14 alters transcriptome and steroid profiles in female green-spotted puffer (Dichotomyctere nigroviridis)
Scientific Reports (2022)
-
Phoenixin-14 Ameliorates Cellular Senescence Against Morphine in M17 Neuronal Cells
Neurotoxicity Research (2022)
-
Linking mitochondrial dynamics and fertility: promoting fertility by phoenixin through modulation of ovarian expression of GnRH receptor and mitochondrial dynamics proteins DRP-1 and Mfn-2
Pflügers Archiv - European Journal of Physiology (2022)
-
Characterization of the G protein-coupled receptor family SREB across fish evolution
Scientific Reports (2021)