Abstract
Moxifloxacin (MX) is an 8-methoxyquinolone antimicrobial drug, which is often used as a positive control in thorough QT (TQT) studies. In the present study we established the population pharmacokinetics model of MX and the relationship of MX concentrations with the QT and various corrected QT (QTc) intervals, and compared the results with other ethnicities. The MX data used for modeling were obtained from a published TQT interval prolongation study of antofloxacin with MX as the positive control. In this four-period crossover study, 24 adult Chinese healthy volunteers received either 200 or 400 mg of oral antofloxacin once daily, 400 mg of MX, or a placebo. Population concentration-effect models were used to investigate the relationship between MX concentrations and QT interval prolongation, baseline-adjusted QTc (ΔQTc), or ΔQTc adjusted with time-matched placebo corrections (ΔΔQTc). The influencing factors of MX PK and the concentration-QTc relationship were determined through covariate screening. Simulation studies were conducted in R2.30 by using the final model with the estimated population mean and intra-individual and inter-individual variability. The estimated pharmacokinetic parameters and the estimated slope of the MX concentration-QT/ΔQTc/ΔΔQTc relationship were described using models and were compared to results for other ethnicities from the literature. We showed that the population pharmacokinetic parameter estimates for total plasma clearance (CL/F), the volume of distribution of central compartment (Vc/F), the distributional clearance in plasma (Q), the volume of distribution of peripheral compartment (Vp/F), and the absorption rate constant (Ka) were 8.22 L/h, 104 L, 3.98 L/h, 37.7 L, and 1.81 1/h, respectively. There was no significant covariate included in the final model. QT interval prolongation of MX estimates ranging from 9.77 to 12.91 ms at the mean average maximum concentration of MX (4.36 μg/mL) and a mean slope ranging from 2.33 to 2.96 ms per μg/mL. In conclusion, no ethnic differences were observed for the MX pharmacokinetic parameters and QT interval prolongation.
Similar content being viewed by others
Introduction
Some non-antiarrhythmic drugs have an undesirable property of delaying cardiac repolarization, which can be measured as QT interval prolongation on a surface electrocardiogram (ECG)1. Delayed cardiac repolarization can introduce the risk of torsade de pointes (TdP). QT interval prolongation on a surface ECG is considered to be a biomarker of proarrhythmia. The design of a thorough QT (TQT) study for non-antiarrhythmic drugs according to the description of the ICH E14 guideline has been optimized in recent years and is typically a randomized, double-blinded, placebo and positive-controlled study conducted in healthy male and female volunteers. Because of the considerable impact of factors influencing the QT interval (eg, heart rate), heart rate-corrected QT interval (QTc) measurements are considered to be the RR interval in ECGs. The primary aim of a TQT study is to assess QT/QTc interval prolongation. The ICH E14 guideline states that the absolute QTc interval prolongation of a drug of more than 450, 480, and 500 ms or a QT interval prolongation change from baseline of 30 ms and 60 ms must be considered when analyzing QT/QTc interval prolongation data2. QT interval prolongation is considered to be positive if the upper bound of the 90% two-sided confidence limit of the ΔΔQTc at the mean maximum concentration (Cmax) for the dataset exceeds 10 ms or an effect on the mean QT/QTc interval exceeds 5 ms2. A positive control is recommended for validating the TQT study results. The conventional corrections of the QT interval include Bazett's (QTcB) and Fridericia's (QTcF) corrections. However, they may not always optimally correct the heart rate QTc3. In addition, population- and individual-corrected QT intervals (QTcP and QTcI, respectively) have been reported to be superior correction factors4. Concentration–QT interval relationship modeling has gained as much attention as the traditional by-time method for analyzing QTc intervals. It can be a more effective method for estimating the QT prolongation of a novel drug during drug development.
Moxifloxacin (MX) is an 8-methoxyquinolone antimicrobial activity drug5. A single oral dose of 400 mg MX is often used as a positive control in TQT studies. MX is metabolized in the liver through sulfate and glucuronide conjugation. Pharmacokinetic (PK) studies conducted in several regions6,7 have revealed ethnic differences in the PK of MX between Japanese and Chinese or German patients. Variations in the genotypes of the metabolizing enzymes can lead to ethnic differences in PK for specific metabolites. However, Kawai8 indicated that the effects of MX did not differ between ethnicities because it was too weak to significantly reduce the clearance of the MX parent compound. In several studies, the QTc prolongation of MX did not significantly differ among Japanese, Korean, and Caucasian patients9,10. However, in another study, the QT effect of MX was found to be higher in Caucasians compared to Africans and Asians11.
The pharmacokinetics of MX and the result of the TQT study may have been affected by ethnic factors. However, it is still unclear whether ethnicity had an effect due to limited data11. Accordingly, we established a population pharmacokinetic model to compare the PK and QT interval along with various types of QTc effects of MX to demonstrate the sensitivity of the study in detecting changes in a mean ΔΔQTc of more than 5 ms or up to 10 ms in a Chinese population. Subsequently, we compared the results to results obtained for other ethnicities.
Materials and methods
Study design and population
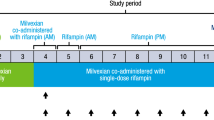
The MX data used for modeling were obtained from a TQT interval prolongation study of antofloxacin with MX as the positive control. A positive prolongation within the normal range without risk of the QT interval caused by antofloxacin has been reported in a conference presentation12. In this four-period crossover study, adult healthy volunteers (n=24) received either 200 or 400 mg of oral antofloxacin once daily, 400 mg of MX, or a placebo13. All the volunteers signed informed consent forms before they were enrolled, and the study protocol was approved by the hospital ethics committee.
Blood- and time-matched ECG samples were collected for PK analysis and QT interval measurements at 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h after treatment on d 1 and d 5. Samples for the trough concentration and time-matched ECG were collected before treatment on d 2, 3 and 4. MX concentrations were measured through a validated high-pressure liquid chromatography (HPLC)-tandem mass spectrometry method (MS/MS) as previously described14. The pre-dose ECG measured at −5 min was considered to be the baseline for the QT interval for the statistical calculations.
Population pharmacokinetic models
Several candidate population pharmacokinetic models, including one and two compartment models with or without lag time, were tested to describe the pharmacokinetics of MX. According to the principle of significant changes in the objective function value (OFV, OFV >6.63, χ2 distribution with 1 degree of freedom) and bootstrap success rate (at least 80%), the final pharmacokinetic model of MX was described as a two compartment model with an absorption rate constant (Ka), total plasma clearance (CL/F), distributional clearance in plasma (Q), and volume of distribution of the central compartment (Vc/F) and peripheral compartment (Vp/F).
Population pharmacokinetic analysis was performed using NONMEM (Version 7.3; GloboMax LLC, Hanover, MD, USA) with PsN 3.4.2. The estimation method was the first-order conditional estimation method (FOCE) with an eta–epsilon interaction. All figures were plotted using R version 2.30 (http://www.r-project.org/). The 95% confidence intervals (CIs) of the 2.5th and 97.5th percentiles of the simulated data for the compartments were compared with the observed data and used to assess the reliability of the final model.
Heart rate-corrected QT and changes in adjusted QTc (ΔQTc, ΔΔQTc)
The QT interval depends strongly on the RR interval. An appropriate correction of QT intervals by RR intervals is essential to obtaining the corrected QT interval, which is used for evaluating drug-induced QT prolongation. In this study, four heart rate correction methods were used to adjust the influence of the RR interval.

Study-specific correction, QTcP=QT/RRβ, where β is the slope of the regression of ln(QT) on ln(RR) for an individual subject with drug-free data in the study population. This calculation indicates that all the subjects have the same power term for the QT correction.
Individual correction, QTcI=QT/RRβi, where β is obtained in the same manner as QTcP but is calculated for each individual. This calculation indicates that the subjects have their own power parameter.
The change in ΔQTc was the QTc interval change from baseline, and the change in ΔΔQTc was ΔQTc of the time-matched difference between MX and placebo.
One-stage concentration–QT interval model
The one-stage concentration–QT interval model, which indicates the raw QT interval, was directly estimated using the observed MX concentration and RR. The relationship between the MX concentration and QT was determined using linear and nonlinear Emax models. The data did not support an Emax model. Therefore, a linear mixed-effect model of concentrations with RR interval correction was selected according to Equation 1:

where intercept represents the intercept of the linear mixed model; Conc and rr are the concentration and RR interval, respectively; slop indicates the slope of the linear mixed effects model; β is the parameter of the RR interval effect; and ɛ is the residual error and is typically normally distributed.
Two-stage concentration–QTc interval model
The two-stage concentration–QT interval model, which indicates the raw QT interval, was corrected according to heart rate; heart rate and baseline correction; or heart rate, baseline, and placebo correction, and then fitted by a linear mixed-effect model of observed concentrations and QTc, ΔQTc, and ΔΔQTc intervals directly by using Equations 2, 3, and 4, respectively.



Statistical model
An exponential error model (Equation 5) and proportional error model (Equation 6) were used to account for the inter-individual variability (IIV) of the PK and PD model, respectively:


where PTV is the parameter estimate for a typical population, Pi is the parameter estimate for the ith individual, ηi are the ith individual random effects and are assumed to be distributed as θ-N (0, ω2).
The additive proportional error model and combined additive and proportional error models were tested in the modeling process by using the following Equations 7, 8, 9:



where Y represents the observation; ipred is the individual predicted concentration or QT/QTc interval; and ɛ1i and ɛ2i are the proportional and additive residual errors, respectively, and each is assumed to be normally distributed as N (0, σ2). Interoccasion variability was ignored because there were no significant changes in the OFV when variability was included in the model.
Covariate model
The collected sex, age, race, weight, height, and body mass index (BMI) data were evaluated as candidate covariates to explain the IIV of parameters in the model estimation. The criterion for a covariate to be added to the final model was a decrease of more than 6.63 in the OFV, which corresponds to a P value of 0.01 (χ2 distribution with 1 degree of freedom). Finally, stepwise backward elimination was conducted. The criteria for a covariate to remain in the final model was P<0.005, and the increase in OFV, which corresponds to a P value of 0.005, is 7.88 (χ2 distribution with 1 degree of freedom) in the OFV.
Model validation
The models were evaluated using visual predictive check (VPC) methods. Additionally, 1000 Monte–Carlo-simulated datasets were obtained from the final model output by using the population mean values and a covariance matrix. A nonparametric bootstrap procedure (1000 replicates) was used to evaluate the final model and parameter estimates.
Concentration–QT interval prolongation simulations
Simulations of 500 new subjects were conducted in R version 2.30 to explore the exposure–response relationship by using the population mean and intra-individual variability and IIV of the parameter estimates of the concentration-QT interval final model. For the simulation, four types of QTc were used.
Results
Subject demographic characteristics
Twenty-four healthy Chinese volunteers received MX (400 mg once daily). Half (n=12) of them were men [mean age (standard deviation), 26.00 (4.41) years]. The mean body weight, height, and BMI of the subjects were 58.58 (6.51) kg, 166.42 (7.47) cm, and 21.17 (1.76) kg/m2, respectively. A total of 539 plasma concentrations were recorded for compartment PK modeling. A total of 537 QT intervals and time-matched heart rates were recorded for concentration–QT interval modeling.
Population pharmacokinetic model
Among all the investigated models, the two-compartment model with first-order absorption most accurately described the population model for the PK of MX. After the demographics were evaluated as candidate covariates to explain the IIV of the parameters, no significant covariate was included in the final model.
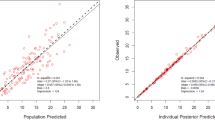
Table 1 summarizes the population final model parameters and bootstrap validation. The estimated values of typical parameters (relative standard error percent, RSE%) for CL/F, Vc/F, Q, Vp/F, and Ka were 8.22 (5.10) L/h, 104 (6.70) L, 3.98 (20.2) L/h, 37.7 (12.1) L, and 1.81 (18.1) 1/h, respectively. This model provided a favorable fit, as shown by the goodness-of-fit plots (Figure 1). VPCs of the final model on d 1 (Figure 2A) and d 5 (Figure 2B) revealed that the data simulated from the final model were consistent with the observed data.
Diagnostic plot of final pharmacokinetic model. (A) Individual predicted concentration versus observed concentration. (B) Population predicted concentration versus observed concentration. (C) Conditional weighted residuals versus population prediction. (D) Conditional weighted residuals versus time. The black line and red line in (A) and (B) represent the line of identity and regression line, respectively, whereas (C) and (D) are the position where the conditional weighted residual equals 0 and the red lines are the regression line.
The visual predictive checks for the pharmacokinetic final model for dose regimens of (A) 1 d and (B) 5 d. Open circles represent observed moxifloxacin concentrations, and the solid and the dashed lines represent the median and the 95% CI of observation, respectively. The middle red shadow areas represent the 95% confidence intervals of the median for the results of a simulation of the final model that was run 1000 times, and the blue shadow areas represent the 95% confidence intervals of the 2.5th and 97.5th percentiles of the results of a 1000 times simulation of the pharmacokinetic final model.
QT categorical analysis
Table 2 summarizes the categorical analysis of QT and QTc (QTcB, QTcF, QTcP, and QTcI) prolongation. The observations of absolute QT prolongation of more than 450 ms with the categorical analysis of the aforementioned factors were 31 (5.77%), 20 (3.72%), 11 (2.05%), 17 (3.17%), and 7 (1.30%), respectively. Moreover, 12 (2.23%) and 3 (0.56%) observations of absolute QT prolongation exceeded 480 and 500 ms with the categorical analysis of QT, respectively, and none of the absolute QTc prolongation changes exceeded these values. The observations of absolute QT, QTcB, QTcF, QTcP, and QTcI prolongation changes from the baseline of more than 30 ms were 82 (15.27%), 28 (5.21%), 17 (3.17%), 20 (3.72%), and 18 (3.35%), respectively. Six (1.12%) observations of absolute QT prolongation were more than 60 ms in the categorical analysis of QT, and none of the absolute QTc prolongation changes from the baseline exceeded 60 ms.
One-stage concentration QT interval model
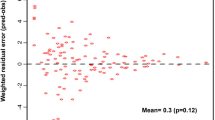
The one-stage concentration QT interval model adequately described the concentration–QT relationship. The diagnostic plots of this model (Figure 3) showed that the population-predicted concentrations, individual-predicted concentrations, and observed concentrations and their closeness to the trend and identity lines as well as the conditional weighted residual value were distributed evenly around zero with most points located between −4 and +4. Figure 4 shows the VPC for the one-stage concentration QT interval final model in dose regimens for d 1 and 5. Most observed concentrations were within the CIs, and the median and 95% confidence interval lines were near the middle of the 1000 results, which suggests that the model exhibited adequate predictive power. Several higher QT points [labeled with an identity (ID) number] were consistently obtained from the same patient (ID=2), which might be related to the higher baseline (484 ms) for this patient.
Diagnostic plot of the final one stage concentration–QT interval model. (A) Individual predicted concentration versus observed concentration. (B) Population predicted concentration versus observed concentration. (C) Conditional weighted residuals versus population prediction. (D) Conditional weighted residuals versus time. The black line and red line in (A) and (B) represent the line of identity and regression line, respectively, whereas in (C) and (D) are the position where the conditional weighted residual equals 0 and the regression lines are red.
The visual predictive checks for the pharmacodynamic final model in dose regimens of (A) the first day and (B) the fifth day. Open circles represent observed QT, and the solid and dashed lines represent the median and the 95% CI of observation, respectively. The middle red shadow areas represent the 95% confidence intervals of the median for the results of 1000 times simulation of the final model, and the blue shadow areas represent the 95% confidence intervals of the 2.5th and 97.5th percentiles of the results of 1000 times simulation of the pharmacodynamic final model. Data points that were judged extreme are labeled with the ID number in this graph.
Table 3 summarizes the results of the parameter estimates and their IIV and the bootstrap of the final one-stage concentration QT interval model. Sex as a significant covariate on Intercept was established as the covariate model. Intercept was described as a function of sex (Equation 10), where θ2 (399 ms) is the population parameter of Intercept, and θIntercept_sex (0.0511) is the parameter of sex effects on intercept, which results in a longer intercept QT for women (20.4 ms) than for men.
Intercept=θ2*(1+θIntercept_sex) (Eq 10)
The estimated β is 0.43. The estimated slope is 2.33 per μg/mL, which indicates a 2.33 ms increase in the QTc interval for every 1 μg/mL increase in the MX concentration. The estimated ΔΔQTcF was 10.16 ms at a mean maximum concentration (Cmax) of 4.36 μg/mL, which exceeded 10 ms. The one-stage concentration QT interval model indicated that MX induces QT prolongation.
Two-stage concentration QT interval model
Table 4 summarizes the parameter and standard error estimates from the two-stage concentration–QT interval model. The point estimation and its one-sided upper limit of 95% CIs of the different methods were calculated at the maximum MX concentration of 400 mg. In the two-stage models with heart rate correction, the estimated slopes of QTcB, QTcF, QTcP, and QTcI were 2.34, 2.24, 2.33, and 2.54 per 1 μg/mL, respectively. The corresponding prolongations of QTcB, QTcF, QTcP, and QTcI at the mean Cmax of MX were 10.20, 9.77, 10.16, and 11.07 ms, respectively. In the two-stage models with heart rate and baseline correction, the estimated slopes of ΔQTcB, ΔQTcF, ΔQTcP, and ΔQTcI were 2.39, 2.27, 2.37, and 2.57 per 1 μg/mL, respectively. The corresponding prolongations of ΔQTcB, ΔQTcF, ΔQTcP, and ΔQTcI at the mean Cmax of MX were 10.42, 9.90, 10.33, and 11.21 ms, respectively. In the two-stage models with heart rate, baseline, and placebo correction, the estimated slopes of ΔΔQTcB, ΔΔQTcF, ΔΔQTcP, and ΔΔQTcI were 2.76, 2.96, 2.95, and 2.91 per μg/mL, respectively. The corresponding prolongations of ΔΔQTcB, ΔΔQTcF, ΔΔQTcP, and ΔΔQTcI at the mean Cmax of MX were 12.03, 12.91, 12.86, and 12.69 ms, respectively.
Figure 5 shows the observed concentration–ΔQTc/ΔΔQTc and the corresponding predicted median with CIs for the results of simulations with 500 new subjects. The 95% CIs of the 2.5th and 97.5th percentiles of the results of simulations with 500 new subjects of the final model were consistent with most of the observed ΔQTc or ΔΔQTc.
Predicted ΔQTc or ΔΔQTc by concentration for simulations with 500 new subjects. Open circles represent observed ΔQTc or ΔΔQTc, the black solid line represents the population prediction, the green dashed line represents the Cmax. The middle red shadow areas represent the 50% confidence intervals of median for the results from simulations with 500 new subjects of final model, and the blue shadow areas represent the 95% confidence intervals of the 2.5th and 97.5th percentiles of the results of simulations with 500 new subjects of the final model.
Discussion
The estimated values of typical parameters (RSE %) for CL/F and Vc/F from the PK model were 8.22 (5.10) L/h and 104 (6.70) L, respectively, which are within the ranges of CL/F (6.7–14.3 L/h) and V/F (62.9–189 L) reported in previous studies15,16,17,18,19,20. In one of those studies, MX concentrations and QTcF data from 20 TQT studies submitted to the Food and Drug Administration (FDA) were analyzed, and no ethnic differences were observed in the PK of MX among Caucasians, blacks, and Asians20.
The observations of MX absolute QT prolongation changes of more than 450, 480, 500 ms or absolute QT prolongation changes from baseline of more than 30 and 60 ms were higher than the measurements obtained in the categorical analysis of the QT correction methods, specifically QTcB, QTcF, QTcP, and QTcI. The MX concentration–QT/QTc relationship was well described by the one- and two-stage linear models with QT correction methods. The results reveal MX as the positive control in the TQT study (mean QT/QTc interval >5 ms and upper bound of the 90% two-sided confidence limit of the ΔΔQTc >10 ms; Table 4). The concentration QT interval models were established by the measured concentration data and time matched QT/QTc. Table 4 indicates that the estimated slopes in this study were 2.24–2.96 ms per 1 μg/mL. The t test revealed no significant difference in the estimated slopes among the various QT correction methods. Estimated QT/QTc/ΔQTc/ΔΔQTc intervals at the mean Cmax of MX were 9.77–12.86 ms, and the one-sided upper limit of 95% CIs of the different methods of MX was 13.90–17.40 ms. In the results of the two-stage models, the ΔΔQTc interval was longer than the QTc and ΔQTc. The estimated QTcF and ΔQTcF intervals (9.77–9.90 ms) were shorter than the intervals obtained from the other QT correction methods in this study (10.16–11.21 ms). However, the estimated ΔΔQTcF interval (12.91 ms) was the longest among all the QT correction methods.
Recent studies have reported the sex- and ethnicity-specific effects of MX on the QTcF interval11,21,22. In one of those studies, the QT effects of MX were greater in Caucasians than in Africans and Asians, and the QTc values were higher in patients with a lower BMI compared to individuals with a higher BMI11. These differences could be related to a simple difference in exposure (PK susceptibility) or the exposure–response relationship (pharmacodynamic sensitivity)11. However, the pooled analysis of 20 TQT studies submitted to the FDA revealed the estimated slopes to be 1.6–4.8 ms per 1 μg/mL with no statistically significant differences between ethnicities. The confidence limits for this comparison were wide20. The estimated slopes in our study were 2.24–2.96 ms per 1 μg/mL, which was within the reported range and near the middle of the range. Moreover, we observed a larger baseline for QT of MX (20.4 ms) in women compared to men, but there was no sex difference in the slope estimates. Therefore, an appropriate concentration–QT model is reliable and can be used in QT risk assessments in TQT studies. In the present study, various QT correction methods for linear models were used to describe the relationship between MX concentrations and QT interval prolongation, which provides reference and comparison data for other studies.
In conclusion, a two-compartment model with first-order absorption and various QT correction methods for linear models fully characterized the time–concentration and concentration–QT interval relationship, respectively. The results of pharmacokinetic parameters and various QT correction methods for linear models are consistent and within previously reported ranges. No ethnic differences were observed in the MX PK and QT interval. In our study, various QT correction methods for linear models were used to describe the relationship between MX concentrations and QT interval prolongation, which may provide a reference and comparison data for future studies.
Author contribution
Kun WANG and Qing-shan ZHENG designed the research; Feng-yan XU and Ji-han HUANG performed the research; Feng-yan XU and Kun WANG wrote the paper; Ying-chun HE, Li-yu LIANG, Lu-jin LI, Juan YANG, Fang YIN and Ling XU helped analyze the data. All authors read and approved the final manuscript.
References
Food and Drug Administration, HHS . International Conference on Harmonisation; guidance on E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs; availability. Notice. Fed Regist 2005; 70: 61134–5.
fda.gov [homepage on the Internet]. E14 implementation working group: ICH E14 guideline: the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs questions & answers (R1). [updated 2016 Mar 3; cited 2017 Jun 23]. Available from: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm323656.htm
Song S, Matsushima N, Lee J, Mendell J . Linear mixed-effects model of QTc prolongation for olmesartan medoxomil. J Clin Pharmacol 2016; 56: 96–100.
Vandemeulebroecke M, Lembcke J, Wiesinger H, Sittner W, Lindemann S . Assessment of QT(c)-prolonging potential of BX471 in healthy volunteers. A 'thorough QT study' following ICH E14 using various QT(c) correction methods. Br J Clin Pharmacol 2009; 68: 435–46.
Sullivan JT, Woodruff M, Lettieri J, Agarwal V, Krol GJ, Leese PT, et al. Pharmacokinetics of a once-daily oral dose of moxifloxacin (Bay 12-8039), a new enantiomerically pure 8-methoxy quinolone. Antimicrob Agents Chemother 1999; 43: 2793–7.
Moise PA, Birmingham MC, Schentag JJ . Pharmacokinetics and metabolism of moxifloxacin. Drugs Today (Barc) 2000; 36: 229–44.
Tanaka T YK, Orii Y, Tsuboi T, Kawano K, Stass H, Tateisi T . Interethnic difference in the pharmacokinetics of a novel quinolone antibiotic, BAY 12-8039 (moxifloxiacin) and estimation of its clinical dose. Jpn J Clin Pharmacol Ther 1999; 30: 231–2.
Hasunuma T, Tohkin M, Kaniwa N, Jang IJ, Yimin C, Kaneko M, et al. Absence of ethnic differences in the pharmacokinetics of moxifloxacin, simvastatin, and meloxicam among three East Asian populations and Caucasians. Br J Clin Pharmacol 2016; 81: 1078–90.
Morganroth J, Wang Y, Thorn M, Kumagai Y, Harris S, Stockbridge N, et al. Moxifloxacin-induced QTc interval prolongations in healthy male Japanese and Caucasian volunteers: a direct comparison in a thorough QT study. Br J Clin Pharmacol 2015; 80: 446–59.
Choi HK, Jung JA, Fujita T, Amano H, Ghim JL, Lee DH, et al. Population pharmacokinetic-pharmacodynamic analysis to compare the effect of moxifloxacin on QT interval prolongation between healthy Korean and Japanese subjects. Clin Ther 2016; 38: 2610–21.
Shah RR . Drug-induced QT interval prolongation: does ethnicity of the thorough QT study population matter? Br J Clin Pharmacol 2013; 75: 347–58.
Lv Y, Hou F, Gao L, Cui H, Wang J, Zhang B, et al. Effects of Antofloxacin on QT/QTc interval and heart rate in healthy subjects. Proceedings of the 10th Chinese Pharmaceutical Congress of Pharmacology; 2010 Aug; Tianjin, China.
Li YF, Wang K, Yin F, He YC, Huang JH, Zheng QS . Dose findings of antofloxacin hydrochloride for treating bacterial infections in an early clinical trial using PK-PD parameters in healthy volunteers. Acta Pharmacol Sin 2012; 33: 1424–30.
Schulte S, Ackermann T, Bertram N, Sauerbruch T, Paar WD . Determination of the newer quinolones levofloxacin and moxifloxacin in plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr Sci 2006; 44: 205–8.
Mason JW . Florian JA Jr, Garnett CE, Moon TE, Selness DS, Spaulding RR. Pharmacokinetics and pharmacodynamics of three moxifloxacin dosage forms: implications for blinding in active-controlled cardiac repolarization studies. J Clin Pharmacol 2010; 50: 1249–59.
Ito F, Ohno Y, Toyoshi S, Kaito D, Koumei Y, Endo J, et al. Pharmacokinetics of consecutive oral moxifloxacin (400 mg/day) in patients with respiratory tract infection. Ther Adv Respir Dis 2016; 10: 34–42.
Peloquin CA, Hadad DJ, Molino LP, Palaci M, Boom WH, Dietze R, et al. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob Agents Chemother 2008; 52: 852–7.
Zvada SP, Denti P, Sirgel FA, Chigutsa E, Hatherill M, Charalambous S, et al. Moxifloxacin population pharmacokinetics and model-based comparison of efficacy between moxifloxacin and ofloxacin in African patients. Antimicrob Agents Chemother 2014; 58: 503–10.
Simon N, Sampol E, Albanese J, Martin C, Arvis P, Urien S, et al. Population pharmacokinetics of moxifloxacin in plasma and bronchial secretions in patients with severe bronchopneumonia. Clin Pharmacol Ther 2003; 74: 353–63.
Florian JA, Tornoe CW, Brundage R, Parekh A, Garnett CE . Population pharmacokinetic and concentration--QTc models for moxifloxacin: pooled analysis of 20 thorough QT studies. J Clin Pharmacol 2011; 51: 1152–62.
Yan LK, Zhang J, Ng MJ, Dang Q . Statistical characteristics of moxifloxacin-induced QTc effect. J Biopharm Stat 2010; 20: 497–507.
Poordad F, Zeldin G, Harris SI, Ke J, Xu L, Mayers D, et al. Absence of effect of telbivudine on cardiac repolarization: results of a thorough QT/QTc study in healthy participants. J Clin Pharmacol 2009; 49: 1436–46.
Acknowledgements
This study was supported by the Scientific Research Fund for Young Teachers (ZYX-QNQD-001), the Shanghai Education Commission (ZY3-CCCX-3-1001), the Special Project for the Priority Academic Program Development of Shanghai Higher Education Institutions (ZYX-CXYJ-014), and the Shanghai University of Traditional Chinese Medicine (2014YSN17).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xu, Fy., Huang, Jh., He, Yc. et al. Population pharmacokinetics of moxifloxacin and its concentration–QT interval relationship modeling in Chinese healthy volunteers. Acta Pharmacol Sin 38, 1580–1588 (2017). https://doi.org/10.1038/aps.2017.76
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2017.76
Keywords
This article is cited by
-
Concentration–response modeling of ECG data from early-phase clinical studies to assess QT prolongation risk of contezolid (MRX-I), an oxazolidinone antibacterial agent
Journal of Pharmacokinetics and Pharmacodynamics (2019)