Abstract
Itching is an intricate, common symptom of dermatologic and systemic diseases, and both TRPV3 and TRPA1 channels have been suggested to function as downstream effector targets. But the relative contributions of TRPV3 and TRPA1 to itch sensation in vivo remain unclear. To dissect the role of TRPA1 or TRPV3 in the cutaneous sensation of itching, we took the advantage of a natural compound carvacrol from oregano, and examined its effect on the induction of scratching behavior in mice. We showed that the intradermal injection of carvacrol (0.01%, 0.1% and 1%, 50 μL) induced scratching in a concentration-dependent manner. But in TRPV3-knockout mice, the scratching induced by carvacrol (1%, 50 μL) was markedly decreased by approximately 64% (from 275 scratching bouts down to 90) within 60 min. Further analysis revealed that TRPV3-knockout caused a reduction of scratching bouts for approximately 40% in the first 20 min (the initial phase), whereas the scratching bouts were reduced by approximately 90% in the last 40 min (the sustained phase). These results were in consistence with those in our whole-cell recordings in HEK-293T cells expressing either TRPA1 or TRPV3: carvacrol exhibited similar potencies in activating either TRPA1 or TRPV3, but carvacrol-activated TRPA1 current showed a rapid desensitization, which was reduced by approximately 90% within 5 min before a complete washout, whereas carvacrol-induced TRPV3 current showed a slow desensitization that caused less than 30% of current reduction in 10 min and left a significant residual TRPV3 current after washout. Our results demonstrate that carvacrol from plant oregano is a skin sensitizer or allergen; TRPV3 is involved in the initial phase and the sustained phase of pruritus, whereas TRPA1 likely contributes to the initial phase.
Similar content being viewed by others
Introduction
Chronic itch (chronic pruritus) is an unmet medical need that manifests as a common symptom of dermatologic and systemic diseases and seriously influences quality of life1,2,3. Although acute itch is often caused in the local affected skin by pruritogens such as histamines, allergens, inflammatory mediators and drugs, chronic itch can also be an indicator of widespread symptoms associated with inflammatory skin diseases, infectious diseases, immune diseases, liver diseases and cancer4. In general, with the exception of histamine-induced itch, there are no accepted effective therapies for chronic itch because of an insufficient understanding of a crucial target that underlies the pathogenesis of itch5,6.
Recent findings have demonstrated that several subtypes of transient receptor potential (TRP) channels play important roles in different types of itch that are induced by pruritogens in rodents (see a recent review by Zhang et al)7. TRPA1 has been demonstrated to regulate both itch transduction and pathophysiological changes in the skin and promote chronic itch8,9. The identification of gain-of-function mutations in human TRPV3 from patients with Olmsted Syndrome, which is characterized by severe itching and skin diseases, indicates the crucial role of TRPV3 channel in itch signaling10,11.
Carvacrol (5-isopropyl-2-methylphenol) is a major component of plant oregano and has been widely demonstrated to be an activator of both TRPV3 and TRPA1 channels with similar potencies12. Carvacrol (250 μmol/L) activates and rapidly desensitizes the TRPA1 current in the continuous presence of the agonist12, whereas TRPV3 is strongly activated and sensitized by carvacrol. Consistent with an increase in intracellular Ca2+ level in response to carvacrol in skin epithelial cells, carvacrol (500 μmol/L) also evokes a slowly developing TRPV3-mediated current (TRPV3) in mTRPV3-expressing HEK293 cells12. However, how and whether carvacrol can sensitize both TRPV3 and TRPA1 by inducing itchy behavior remains unknown. In this study, we examined the effect of intradermal injection of carvacrol on mice and found that natural carvacrol induces scratching behavior primarily by sensitizing TRPV3 in the skin in a concentration-dependent manner.
Results
Intradermal injection of carvacrol induces pruritus in mice
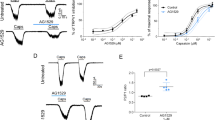
To examine whether the skin sensitizer carvacrol can induce a pruritic effect, we administered an intradermal injection (50 μL) of carvacrol into the right nape of mouse necks. After the injection of carvacrol, the mice were immediately put into an observation box for continuous video recording for 60 min in a quiet room. The number of scratching bouts was determined in 5-min bins for 60 min. The results revealed that the different concentrations of carvacrol (0.01%, 0.1% and 1%) caused a concentration-dependent increase in scratching bouts (17±5; 166±23 and 399±63, n=11 for each group, respectively) compared with the vehicle control (10% ethanol, 50 μL; 15±3, n=11; Figure 1A and 1B). The results demonstrated that carvacrol as a skin allergen induced pruritus in a concentration-dependent manner, which suggests that the activation of either TRPV3 or TRPA1 channel was responsible for the carvacrol-mediated scratching in mice.
Carvacrol induces pruritus in mice in a concentration-dependent manner. (A) The time course of scratching after the intradermal injection (time=0). The number of scratching bouts per 5 min after 50 μL injections of vehicle (n=11) or carvacrol (0.01%, 0.1%, 1%, n=11) was counted. (B) Summary of panel A for a comparison of scratching bouts over 60 min between the four groups. Each value represents the mean±SEM. NS, not significant, *P<0.05, ***P<0.001, ****P<0.0001.
Suppression of carvacrol-induced scratching in TRPV3−/− mice
To further evaluate whether TRPV3 or TRPA1 plays a crucial role in the carvacrol-induced itch sensation, we utilized TRPV3-knockout mice and tested the effect of carvacrol on scratching bouts within 60 min. As demonstrated in Figure 2A and 2B, the injection of carvacrol (0.1%) into the right nape of TRPV3−/− mouse necks resulted in a dramatic decrease in scratching bouts of approximately 64% to 90±7 (n=11) compared with 248±10 (n=8) in TRPV3+/+ mice. The analysis of carvacrol-induced scratching revealed two phases, the first 20 min and the last 40 min (Figure 2C and 2D). Silencing TRPV3 only caused a reduction of scratching bouts for approximately 40% in the first phase (Figure 2C). By contrast, the scratching bouts were reduced by approximately 90% in the second phase (Figure 2D). These results suggested a major role of TRPV3 in pruritus.
Knocking out TRPV3 suppresses scratching behaviors induced by carvacrol. (A) The time course of scratching after the intradermal injection (time=0) in C57BL/6J with TRPV3+/+ and TRPV3−/− mice. The number of scratching bouts per 5 min after 50 μL injection of carvacrol group (1%, n=8) was counted. (B) Summary from panel A, a comparison of the scratching bouts within 60 min between the groups of WT mice (248±10, n=8) and TRPV3−/− mice (90±7). Each value represents the mean±SEM, n=8 mice. ****P<0.0001, Student's t-test, compared with TRPV3+/+ mice. (C) A comparison of scratching bouts during the first 20 min between the groups of WT mice (137±10, n=8) and TRPV3−/− mice (82±7, n=8). (D) A comparison ofscratching bouts within the last 40 min between the groups of WT mice (110±16, n=8) and TRPV3−/− mice (9±2, n=8). Each value represents the mean±SEM, n=8 mice. ***P<0.001, ****P<0.0001, Student's t-test, compared with TRPV3+/+ mice.
To further confirm the effect of carvacrol on scratching, we performed whole-cell patch clamp recordings of TRPV3 or TRPA1 expressing HEK-293T cells. Bath application of carvacrol (250 μmol/L) elicited a robust activation and a quick desensitization of the TRPA1 current, which resulted in current reduction of approximately 90% within 5 min before a complete washout after the application of carvacrol (Figure 3A). By contrast, the addition of carvacrol (250 μmol/L) evoked a slow activation of TRPV3 before a slow desensitization and caused only less than 30% of a current reduction in 10 min with a significant residual TRPV3 current after washout (Figure 3B). This result is consistent with the observation that the first phase of pruritus within the 20 min was mediated by activation of both TRPV3 and TRPA1, whereas during the second phase of carvacrol-induced scratching, TRPV3-mediated pruritus significantly accounted for more than 90% of the effect (Figure 4). This pattern of scratching induced by carvacrol suggests a major and pivotal role for TRPV3 in itch sensation that is consistent with the observations of the sustained activation of TRPV3 and rapid desensitization of TRPA1 current activated by carvacrol.
Activation and desensitization of TRPA1 or TRPV3 currents by whole-cell patch clamp recordings in response to carvacrol. (A) The representative TRPA1 current was activated and desensitized rapidly by carvacrol (250 μmol/L) (n=3). (B) The representative TRPV3 current was activated and slightly desensitized by carvacrol (250 μmol/L) compared with TRPA1 from panel A (n=3).
A proposed model for the contributions of TRPV3- and TRPA1-mediated pruritus induced by agonist carvacrol. The total pruritus effect mediated by carvacrol is composed of TRPV3-mediated (PTRPV3) and TRPA1-mediated (PTRPA1) components. The calculation was based on the equation that defines the Ptotal=PTRPA1+PTRPV3. The total pruritus effect was divided into two phases within the 60 min: Phase 1 for first 20 min and Phase 2 for the last 40 min. The first phase of pruritus was mediated by activation of both TRPV3 and TRPA1 channels with TRPV3 accounting for 40% of area of under curve (AUC) of the total effect. In phase 2, the TRPV3-mediated pruritus accounted for approximately 90.2% of the total pruritus effect.
Discussion
To date, both TRPV3 and TRPA1 have been indicated as downstream targets for itch sensations3,13. As such, there is much interest in understanding which channel plays a crucial role in the itch sensation and how pharmacological intervention or inhibition of both targets can achieve better itch therapy. In this study, our findings demonstrate that natural carvacrol, the agonist of TRPA1 and TRPV3, can cause drastic itch sensations and that knocking out TRPV3 suppresses approximately 64% of itch behavior in mice. The total pruritus effect contains both TRPV3-mediated and TRPA1-mediated elements of itch sensation.
The pattern of scratching induced by carvacrol suggests a major and pivotal role for TRPV3 in itch sensation, which is also consistent with the observations of rapid desensitization of TRPA1 current induced by carvacrol (see data from Figure 3). According to our results, TRPV3 is involved in the initial phase and the sustained phase of pruritus, whereas TRPA1 likely contributes the initial phase. This notion is consistent with the previous observations that carvacrol activates TRPA1 before rapidly desensitizing the current12, and contrasts the notion that carvacrol-induced desensitization of TRPV3 is significantly delayed and reduced, and thus the inhibition of TRPV3 may achieve more effective itch therapy.
Skin allergies frequently occur in daily life when people come in contact with natural components from plants, such as carvacrol, eugenol and thymol. In this study, our findings also demonstrate that carvacrol induces pruritus and functions as a skin sensitizer and allergen. Therefore, caution should be taken when carvacrol or monoterpene chemicals acting on either TRPV3 or TRPA1 channel are used as cosmetic products.
Materials and methods
Cell Culture and Transfection
HEK-293T cells were maintained at 37 °C in media containing 90% Dulbecco's modified Eagle's medium and 10% fetal bovine serum in 5% CO2. HEK-293T cells were plated onto glass coverslips for subsequent patch clamp recordings. Transient transfections of 2 μg human TRPV3 cDNA in pIRES2-EGFP or human TRPA1 cDNA in pcDNA4-TO were made using Lipofectamine 2000 (Invitrogen). Whole-cell patch clamp recordings were performed between 24 and 48 h after transfection.
Animals
Male C57BL/6 mice (6–8 weeks, 18–22 g, Beijing Vital River Laboratory Animal Technology Company) and TRPV3−/− mice (provided by Dr Yang Y) were used for the behavioral evaluations. TRPV3−/− mice were generated by deleting the essential exons that encoded the putative pore region and adjacent transmembrane segments (S5 and S6) of TRPV3. All mice were kept in a temperature-controlled environment (23–25 °C) with daylight between 8:00–22:00 with free access to food and water. For the establishment of the carvacrol pruritogenic model in the right nape of the mouse neck, each mouse neck was clipped and depilated with electric hair clippers 24 h before beginning the experiments. All experiments were performed under the guidelines and regulations of Qingdao University on the management of laboratory animals.
Drugs
Carvacrol was purchased from Sigma-Aldrich Corp and was stored at 4 °C daily. Due to carvacrol being almost insoluble in normal saline, all the different concentrations of carvacrol were dissolved in 10% ethanol before further dilutions and combined with ultrasound to promote its emulsion. Ethanol was purchased from Sinopharm Chemical Reagent Co, Ltd, and stored in a cool and airy place.
Whole-cell patch clamp recordings
Currents expressed in HEK-293T cells were recorded using a HEKA EPC10 amplifier with PatchMaster software (HEKA, HEKA Instrument Inc, Lambrecht/Pfalz, Germany). Patch pipettes were pulled with borosilicate glass using a puller (DMZ-Universal) and fire-polished to a resistance of 3–5 megohms. The bath solution contained 140 mmol/L NaCl, 2 mmol/L MgCl2, 2 mmol/L CaCl2, 5 mmol/L KCl, 10 mmol/L glucose, and 10 mmol/L HEPES, pH 7.3. The pipette solution contained 140 mmol/L KCl, 5 mmol/L EGTA and 10 mmol/L HEPES, pH 7.3. The membrane potential was held at 0 mV, and the current in response to a 400-ms step to 80 mV, followed by a 400-ms step to 80 mV at 1-s intervals. All recorded data were analyzed with Igor Pro (Wave-metrics) and Origin 8.6 (OriginLab).
Behavioral tests
Before each experiment, the mice were placed in an observation box (9 cm×9 cm×13 cm) for approximately 30 min for adaptation. After intradermal injection of the drug, the mice were immediately put into the observation box under continuous video recording for a period of 60 min in a quiet room. The number of scratching bouts was recorded at 5-min intervals as a time unit. One bout of scratching was defined as the mouse lifting its paw towards the injection site to scratch until the paw was returned to the floor.
Data analysis
All data are expressed as the mean±SEM. Statistical differences between the vehicle and the different concentrations of carvacrol groups were assessed by a one-way ANOVA. The statistical significance of two different genotypes of mice was evaluated using unpaired Student's t-test for the data.
References
Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M . The neurobiology of itch. Nat Rev Neurosci 2006; 7: 535–47.
Patel T, Yosipovitch G . The management of chronic pruritus in the elderly. Skin Ther Lett 2010; 15: 5–9.
Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, et al. The ion channel TRPA1 is required for chronic itch. J Neurosci 2013; 33: 9283–94.
Stander S, Weisshaar E, Mettang T, Szepietowski JC, Carstens E, Ikoma A, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol 2007; 87: 291–4.
Mishra SK, Hoon MA . The cells and circuitry for itch responses in mice. Science 2013; 340: 968–71.
Storan ER, O'Gorman SM, McDonald ID, Steinhoff M . Role of cytokines and chemokines in itch. Handb Exp Pharmacol 2015; 226: 163–76.
Zhang X . Targeting TRP ion channels for itch relief. Naunyn Schmiedebergs Arch Pharmacol 2015; 388: 389–99.
Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 2009; 139: 1353–65.
Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 2011; 14: 595–602.
Lai-Cheong JE, Sethuraman G, Ramam M, Stone K, Simpson MA, McGrath JA . Recurrent heterozygous missense mutation, p.Gly573Ser, in the TRPV3 gene in an Indian boy with sporadic Olmsted syndrome. Br J Dermatol 2012; 167: 440–2.
Lin ZM, Chen Q, Lee MY, Cao X, Zhang J, Ma DL, et al. Exome sequencing reveals mutations in TRPV3 as a cause of olmsted syndrome. Am J Hum Genet 2012; 90: 558–64.
Xu H, Delling M, Jun JC, Clapham DE . Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 2006; 9: 628–35.
Yamamoto-Kasai E, Imura K, Yasui K, Shichijou M, Oshima I, Hirasawa T, et al. TRPV3 as a therapeutic target for itch. J Invest Dermatol 2012; 132: 2109–12.
Acknowledgements
We are grateful to Dr Y YANG at Peking University First Affiliated Hospital who provided the TRPV3-deficit mice, and Ting-ting CUI wishes to thank Q GAO and YZ MIAO for their help in the laboratory. This work was supported by research grants to KeWei WANG from the National Natural Science Foundation of China (81573410), the Ministry of Science and Technology of China (2014ZX09507003-006-004) and the Natural Science Foundation of Shandong Province (ZR2015QL008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, Tt., Wang, Gx., Wei, Nn. et al. A pivotal role for the activation of TRPV3 channel in itch sensations induced by the natural skin sensitizer carvacrol. Acta Pharmacol Sin 39, 331–335 (2018). https://doi.org/10.1038/aps.2017.152
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2017.152
Keywords
This article is cited by
-
Involvement of skin TRPV3 in temperature detection regulated by TMEM79 in mice
Nature Communications (2023)
-
FRET analysis of the temperature-induced structural changes in human TRPV3
Scientific Reports (2023)
-
Structural mechanism of TRPV3 channel inhibition by the anesthetic dyclonine
Nature Communications (2022)
-
TRPV3 enhances skin keratinocyte proliferation through EGFR-dependent signaling pathways
Cell Biology and Toxicology (2021)