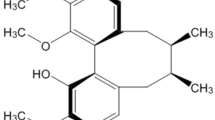
Abstract
Aim:
It is general believed that mitochondrial dysfunction and oxidative stress play critical roles in the pathology of Parkinson's disease (PD). Dihydromyricetin (DHM), a natural flavonoid extracted from Ampelopsis grossedentata, has recently been found to elicit potent anti-oxidative effects. In the present study, we explored the role of DHM in protecting dopaminergic neurons.
Methods:
Male C57BL/6 mice were intraperitoneally injected with 1-methyl4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) for 7 d to induce PD. Additionally, mice were treated with either 5 or 10 mg/kg DHM for a total of 13 d (3 d before the start of MPTP, during MPTP administration (7 d) and 3 d after the end of MPTP). For the saline or DHM alone treatment groups, mice were injected with saline or DHM for 13 d. On d 14, behavioral tests (locomotor activity, the rotarod test and the pole test) were administered. After the behavioral tests, the mice were sacrificed, and brain tissue was collected for immunofluorescence staining and Western blotting. In addition, MES23.5 cells were treated with MPP+ and DHM, and evaluated using cell viability assays, reactive oxygen species (ROS) measurements, apoptosis analysis and Western blotting.
Results:
DHM significantly attenuated MPTP-induced mouse behavioral impairments and dopaminergic neuron loss. In the MES23.5 cells, DHM attenuated MPP+-induced cell injury and ROS production in a dose-dependent manner. In addition, DHM increased glycogen synthase kinase-3 beta phosphorylation in a dose- and time-dependent manner, which may be associated with DHM-induced dopaminergic neuronal protection.
Conclusion:
The present study demonstrated that DHM is a potent neuroprotective agent for DA neurons by modulating the Akt/GSK-3β pathway, which suggests that DHM may be a promising therapeutic candidate for PD.
Similar content being viewed by others
Introduction
Parkinson's disease (PD) is an age-related neurodegenerative disease characterized by progressive depletion of dopamine due to selective loss of dopaminergic neurons in the substantia nigra pars compacta (SNc)1,2. Although the precise pathogenic mechanism leading to neurodegeneration in PD is unknown, it is widely accepted that neuroinflammation, oxidative stress (OS) and mitochondrial dysfunction may contribute to dopaminergic neuron damage3,4,5,6,7. In addition to dopamine replacement therapy, such as levodopa, in past decades, tremendous efforts have been made to develop new therapeutic agents for PD, including selective dopamine receptor agonists, A2A receptor antagonists and dual dopamine/serotonin receptor agonists8,9,10,11,12. However, these drugs can relieve only the symptoms of PD and are not a cure. Therefore, identifying potent and effective neuroprotective agents to stop progressive dopaminergic neuron loss remains an important challenge in the treatment of PD.
Dihydromyricetin (DHM) is a flavonoid extracted from Ampelopsis grossedentata in southern China. DHM has been shown to be a potent anti-oxidative agent and to protect mitochondrial functions13,14. In addition, recent studies have reported that DHM has anti-tumor and anti-inflammatory effects15,16,17,18. Interestingly, DHM can also protect PC12 cells from oxidative stress and elicit beneficial effects in both Alzheimer's disease and alcohol intoxication13,19,20. Although the molecular mechanisms underlying DHM-mediated effects have not been elucidated, studies have shown that the AMPK/GLUT4 signaling pathway may contribute to DHM-mediated inhibition of the oxidative stress response. In addition, the NF-κB/MAPK signaling pathway and p53 have also been suggested to be associated with the anti-inflammatory and anti-tumor effects of DHM, respectively13,15,21. In the present study, we aimed to explore the potential therapeutic role of DHM in a mouse model of Parkinson's disease. The results indicated that administration of DHM significantly restored the MPTP-induced behavioral impairments. We further demonstrated that DHM modulated the glycogen synthase kinase-3β (GSK-3β) pathway, which may contribute to DHM-mediated dopaminergic neuronal protection.
Materials and methods
Drugs and reagents
DHM was obtained from Shanghai Standard Biotech Co, Ltd (Shanghai, China) and dissolved in dimethylsulfoxide (DMSO) to a concentration of 1 mg/μL and then diluted with saline for intraperitoneal (ip) injection (5 or 10 mg/kg) of the mice. The final DMSO concentration was below 0.1%, and the injection volume was 1 mL/kg of body weight. For cell culture, DHM was solubilized using DMSO to a 100 mmol/L stock solution and kept at −20 °C. The stock solution was diluted with cell culture medium to designated concentrations between 5 μmol/L and 100 μmol/L. MPTP (Sigma-Aldrich, #M0896) was dissolved in saline. Mice were intraperitoneally injected with 25 mg/kg MPTP. MPP+ (Sigma-Aldrich, #D048) was dissolved in PBS and added to the cells for a final concentration of 500 μmol/L22. LY294002 was purchased from Beyotime (Shanghai, China) and stored at a concentration of 10 mg/mL, and the final concentration in cell culture was 30 μmol/L.
Animals and MPTP administration
Male C57BL/6J mice (6–8 weeks, 20–25 g) were purchased from the Shanghai SLAC Laboratory Animals Co Ltd (Shanghai, China). The animals were housed in SPF conditions (temperature: 21±1 °C) with air exchange every 20 min and an automatic 12 h/12 h light-dark cycle (lights on from 6:00 to 18:00). Animals were allowed free access to a standard laboratory diet and water. All animal care and experimental protocols were approved by the Institutional Animal Care and Use Committee of Soochow University and were in compliance with the Guidelines for the Care and Use of Laboratory Animals (Chinese-National-Research-Council, 2006) and with the 'ARRIVE' guidelines (Animals in Research: Reporting In Vivo Experiments). Male C57BL/6 mice were divided into five groups: saline, DHM alone (10 mg/kg), MPTP (25 mg/kg), DHM (5 mg/kg)+MPTP (25 mg/kg) and DHM (10 mg/kg)+MPTP (25 mg/kg). Mice were intraperitoneally injected with MPTP for 7 d. For the groups with MPTP and DHM treatment, mice were given ip 5 or 10 mg/kg DHM for a total of 13 d (3 d before the start of MPTP, during MPTP administration (7 d) and three days after the end of MTPT). On d 14, behavioral tests were conducted. After the behavioral tests, the mice were sacrificed and the brain tissue was collected for further analysis22.
Behavioral testing
Locomotor activity
Mouse horizontal locomotor activity was measured using an infrared photobeam activity cage system (Jiliang Ltd, Shanghai, China). Before the experiment, the animals were habituated in the test room for 30 min. Then, mice were individually placed in the center of a Plexiglas/polyvinyl chloride (PVC) cage (25 cm×25 cm×30 cm). Total ambulatory counts and the distance travelled (cm) were recorded in a 50 min period23.
Rotarod test
One day before testing, the mice were trained until they could remain on the rotarod (UGO Basile, Italy) for 120 s without falling. During testing, mice were placed on the rotarod, and the rotation speed was increased from 5 to 40 r/min in 5 min. The latency (time) to fall from the rotarod was automatically recorded. Each mouse was tested in three independent trials with a 20 min intertrial interval. The latency to fall was calculated as the average of three trails24,25.
Pole test
Mice were placed head down on the top of a vertical wooden pole (60 cm in length and 2 cm in diameter) with a rough surface. The latency for the mice to climb down from the top of the pole to the base was measured. Trials were considered a failure if the mouse jumped or slid down the pole. Each mouse underwent five trials, and the average latency was then recorded25.
Cell culture
MES23.5 cells were kindly provided by Dr Wei-dong LE (Baylor College of Medicine, Houston, TX, USA). The cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) (Gibco, 12430-054) containing 5% fetal bovine serum (FBS) (Gibco, 16000-044), 1% penicillin/streptomycin (Invitrogen, 15140-122) and insulin-transferrin-selenium (1:100 dilution, Gibco, 1672743) at 37 °C in a humid environment (5% CO2, 95% air). Cells were seeded at a density of 1×105/cm2, and treated with MPP+ and DHM for 24 h for cell viability assays and ROS measurement and 48 h for apoptosis analysis.
Immunofluorescence staining
Following the behavioral tests, mice were anesthetized with 4% chloral hydrate and then perfused through the left ventricle perfusion with phosphate-buffered saline (PBS, pH 7.4), followed by paraformaldehyde (4%) in 0.1 mol/L PBS (pH 7.4). Following perfusion, the mice were sacrificed, and their brains were collected and post-fixed in paraformaldehyde overnight at 4 °C, followed by 30% sucrose overnight at 4 °C. The brains were serially cut into 20 μmol/L coronal sections using a freezing microtome. For immunofluorescence staining23, the midbrain sections were rinsed three times with 0.1 mol/L PBS and incubated in PBS containing 3% BSA and 0.3% Triton for 2 h at room temperature. The sections were washed in PBS and then incubated with anti-TH antibody (1:400, Millipore, USA) at 4 °C for 24 h. The sections were washed (3×10 min) in PBST (PBS containing 0.3% Triton) and incubated with Alexa Fluor 488 conjugated goat anti-rabbit IgG (1:400, Thermo Fisher Scientific) for 2 h at room temperature in the dark. The sections were washed (10 min each) and then mounted using mounting medium (Thermo Fisher Scientific, USA) and imaged using a laser confocal fluorescent microscope.
MTT assay
MES23.5 cells were seeded in 96-well plates at a density of 4000 cells per well. After 12 h, 500 μmol/L of MPP+ and different concentrations of DHM (DHM was added 30 min before MPP+) were added to the wells (three replicates for each group). Briefly, the cells were rinsed with PBS, and 30 μL MTT (0.5 mg/mL) was added to each well. The plates were incubated at 37 °C for an additional 2 h. Then, 100 μL of DMSO was added to each well, and the plates were shaken using a microplate shaker to completely dissolve the MTT formazan crystals. The absorbance was measured at 570 nm using a spectrophotometer26.
Apoptosis detection
Apoptosis was measured using flow cytometry with an Annexin V-FITC Apoptosis Detection Kit (Beyotime, Shanghai, China)26,27. The cells were digested with trypsin and then washed and resuspended in cold PBS at a density of 1×105. The cells were centrifuged at 1000 r/min for 5 min and resuspended with 195 μL of Annexin V-FITC combing solution. Then, the cells were incubated in 5 μL of Annexin V-FITC and 10 μL of propidium iodide at room temperature for 20 min in the dark. After incubation, the percentage of apoptotic cells was measured using flow cytometry.
Measurement of intracellular ROS
Cells were seeded at a density of 1×105 and treated with MPP+/DHM, as described above. CellROXTM Deep Red Reagent (Life Technologies, USA) was added to the cells at a final concentration of 5 μmol/L. The cells were incubated for 30 min at 37 °C, and then the culture medium was removed, and the cells were washed three times with PBS. The cells were digested with trypsin and then resuspended in PBS. Intracellular ROS were determined using flow cytometry26.
Western blotting
Cells or tissues were lysed in RIPA buffer (Cell Signaling Technology, USA) and incubated at 95 °C for 5 min. Protein concentrations were determined using a Bradford Protein Assay Kit (Bio-Rad, Hercules, CA, USA). Proteins were separated on sodium dodecyl sulfate polyacrylamide gel and then transferred to a polyvinylidene difluoride membrane. Membranes were probed with anti-TH antibody (1:1000, Millipore), anti-VMAT2 antibody (1:500 Santa Cruz), anti-GSK3β antibody (1:1000, Cell Signaling Technology), anti-p-GSK3β antibody (1:1000, Cell Signaling Technology), anti-Akt antibody (1:600, Cell Signaling Technology) or anti-p-Akt antibody (1:400, Cell Signaling Technology). Membranes were probed with monoclonal anti-α-tubulin antibody (1:10 000, Sigma-Aldrich) as a loading control. The results were analyzed using a ChemiScope 3300 Mini (Clinx Science Instruments, Shanghai, China).
Statistical analysis
Data were analyzed using Graph Pad Prism software (version 6.0) and are expressed as the mean±standard error of the mean (SEM) in behavial test and mean±SD in other experiment. Two-way and one-way ANOVAs followed by Bonferroni post hoc tests were utilized for multiple-group comparisons. Student's t-test was used for comparisons between two groups; P<0.05 was considered statistically significant.
Results
DHM promotes recovery from MPTP-induced motor impairment
We first examined the effects of DHM administration on experimental Parkinsonism in a mouse model. As expected, MPTP treatment induced severe motor impairments, as evidenced by the climbing pole and rotarod tests. Administration of DHM (5 or 10 mg/kg) attenuated the MPTP-induced deficit in movement balance (Figure 1A and 1B). Similarly, DHM also improved exploratory and locomotor activity in MPTP-treated mice (Figure 1C and 1D). DHM treatment alone had no effect on mouse behavior compared with the saline group (Figure 1). These results suggest that DHM elicits a potent protective effect against MPTP-induced impairments in motor function. In addition, DHM administration significantly attenuated the MPTP-induced decrease in tyrosine hydroxylase (TH) expression in the substantia nigra pars compacta (SNc) (Figure 2). To further confirm this observation, we used Western blotting to measure the expression level of the DA neuron markers, TH and vesicular monoamine transporter 2 (VMAT2), in another set of animals with identical treatments, as described in Figure 1. As shown in Figures 3 and 4, DHM treatment significantly restored the MPTP-induced decrease in TH and VMAT2 expression in the striatum and SNc. DHM alone did not alter the expression of TH or VMAT2 (data not shown). Furthermore, DHM also restored the MPP+-induced impairment in TH and VMAT2 expression in a dopamine neuronal MES 23.5 cell line (Figures 3 and 4). Taken together, these data demonstrate that DHM elicits a potent neuroprotective effect on dopamine neurons.
Chronic treatment with DHM induces behavioral motor recovery. Male C57BL/6 mice were divided into five groups: saline, 10 mg/kg DHM, 10 mg/kg MPTP, 5 mg/kg DHM+MPTP and 10 mg/kg DHM+MPTP. Mice were injected intraperitoneally (ip) with MPTP (25 mg/kg) for 7 d. For the groups treated with both DHM and MPTP, mice were given 5 or 10 mg/kg of DHM for a total of 13 d (3 d before the start of MPTP, during MPTP administration (7 d) and 3 d after the end of MPTP). For the saline or DHM alone groups, mice were injected with saline or DHM for 13 consecutive days. On d 14, behavioral tests were conducted as described in Methods. (A) Climbing pole test; (B) Rotarod test. The results are expressed as the total time that the mouse remained on the pole or rotarod. (C) Average travel speed; (D) Total distance travelled. The total distance and average speed were measured using the open field test with a video-based system, as described in Methods. The results are presented as the mean±SEM of at least 12 mice per group. Statistical analyses were performed using a one-way ANOVA (*P<0.05, **P<0.01).
DHM treatment attenuated the MPTP-induced decrease in tyrosine hydroxylase (TH) expression in mice. Animals treated as described in Figure 1 were sacrificed following behavioral testing. Tissue preparation and immunofluorescence staining with anti-TH antibody were conducted as described in Methods. Representative photomicrographs are shown. (A) ×10 magnification. (B) ×40 magnification of the substantia nigra area.
DHM alleviates the MPTP/MPP+-induced decrease in TH expression in vivo and in cultured MES23.5 DA neurons. Mice were treated as described in Figure 1. The striatum and SNc were dissected and processed as described in Methods. (A) TH expression in the striatum. (B) TH expression in the SNc. (C) MES23.5 DA cells were cultured as described in Methods and treated with MPP+ or vehicle in the presence or absence of various concentrations of DHM for 24 h. Cells were collected for Western blotting using anti-TH antibody (1:1000, Millipore). TH, tyrosine hydroxylase. Mean±SD. n=3. *P<0.05, **P<0.01.
DHM alleviates the MPTP/MPP+ induced decrease in VMAT2 expression in vivo and in cultured MES23.5 DA cells. Mice were treated as described in Figure 1. The striatum and SNc were dissected and processed as described in Methods. (A) VMAT2 expression in the striatum. (B) VMAT2 expression in the SNc. (C) MES23.5 cells were cultured as described in Figure 3 and were collected for Western blotting using anti-VMAT2 antibody (1:500, Santa Cruz). VMAT2, Vesicular monoamine transporter 2. Mean±SD. n=3. *P<0.05, **P<0.01.
DHM protects DA neurons from MPP+ toxicity and inhibits the production of ROS in MES23.5 cells
We next investigated the effect of DA neuronal protection in MES23.5 cells. We found that DHM treatment alone did not alter cell viability at concentrations up to 100 μmol/L in cultured MES23.5 cells (data not shown). However, using an MTT assay, we observed that DHM attenuated MPP+-induced cell injury in a dose-dependent manner (Figure 5A). The neuroprotective effect of DHM was further confirmed using flow cytometry (Figure 5C). It is well known that MPP+ inhibits mitochondrial complex I enzyme activity and leads to increased production of ROS. Several studies have showed that DHM is a powerful ROS scavenger14,28. Therefore, we examined whether DHM also inhibited FFF+-induced production of ROS. As shown in Figure 5B, DHM significantly inhibited the MPP+-induced production of ROS in MES23.5 cells.
DHM increased cell viability, inhibited apoptosis and inhibited ROS production in cultured DA neurons treated with MPP+. MES23.5 DA cells were cultured as described in Methods and treated with either MPP+ or vehicle in the presence or absence of different concentrations of DHM for 24 h. (A) Cell viability as measured using an MTT assay. (B) ROS generation was determined using flow cytometry after the cells were stained with CellROXTM Deep Red Reagent (Life technologies, USA) for 30 min at 37 °C. (C) The percentage of apoptotic cells was detected using flow cytometry. A total of 1×105 MES23.5 DA cells were cultured with both MPP+ and DHM for 48 h. The cells were stained with Annexin V and PI for 20 min and detected using flow cytometry (Annexin V-FITC Apoptosis detection kit, Beyotime, China). Mean±SD. n=3. **P<0.01.
DHM inhibition of GSK-3β activity may contribute to DA neuronal protection
The phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt/PKB) signaling pathway plays a key role in cell survival, proliferation and differentiation29,30,31. Akt is a direct downstream effector of the PI3K pathway and is activated by phosphorylation at Ser473. Akt phosphorylates its downstream substrate glycogen synthase kinase-3β (GSK-3β), which leads to inactivation of the enzyme. GSK-3β dysregulation plays an important role in the pathogenesis of many neurodegenerative diseases, including Parkinson's disease32,33,34. Alterations in GSK-3β activity have been shown to contribute to DA neuronal death induced by MPTP and other insults. Therefore, we investigated the role of DHM in the Akt/GSK-3β pathway. In cultured MES23.5 DA cells, DHM alone activated Akt and increased the phosphorylation of GSK-3β at ser-9 in a time- and dose-dependent manner (Figure 6A and B). As expected, MPP+ treatment induced activation of GSK-3β, whereas DHM pretreatment abolished the MPP+-induced activation of GSK-3β (Figure 6C). Accordingly, DHM restored MPP+-induced suppression of TH expression. Inhibition of Akt activation using a PI3K/Akt selective inhibitor (LY294002) abolished the DHM-induced restoration of TH expression (Figure 6D). These results indicate that DHM regulation of the GSK-3β pathway may contribute to the DA neuroprotective effects of DHM. Finally, we further investigated the regulation of GSK-3β by DHM in vivo. MPTP treatment activated GSK-3β in both the striatum and SNc (Figure 7A and 7B). However, DHM pretreatment attenuated the MPTP effect (Figure 7A and 7B). Taken together, our data suggest that DHM protects DA neurons from MPTP-induced lesions by modulating GSK-3β activity.
DHM attenuated MPP+-induced GSK-3β activation and restored MPP+-induced suppression of TH expression. MES23.5 DA cells were cultured as described in Methods. The expression of AKT, p-AKT, GSK-3β, p-GSK-3β and TH was accessed using Western blotting. (A) Cells were treated with DHM alone at different concentrations (0, 10, 25, and 50 μmol/L) for 30 min. (B) Cells were treated with 25 μmol/L of DHM and collected at 0, 10, 30, 60, and 90 min. **P<0.01. (C) Cells were pretreated with/without DHM for 30 min prior to and during MPP+ treatment for an additional 1 h. *P<0.05 vs control. ##P<0.01 vs MPP+. (D) Cells were treated with MPP+ or vehicle in the presence or absence of DHM and LY294002 for 24 h. Data are expressed as the mean±SD. n=3. Two-way ANOVA. **P<0.01.
DHM attenuated MPTP-induced GSK-3β activation in both the striatum (A) and SNc (B). Male C57BL/6 mice were divided into four groups: saline, 25 mg/kg MPTP, 10 mg/kg DHM and 10 mg/kg DHM+25 mg/kg MPTP. For the groups with DHM treatment, mice were given 10 mg/kg DHM (ip) daily for a total of 13 d (3 d before the start of MPTP, during MPTP administration (7 d) and 3 d after the end of MPTP). The striatum and SNc were dissected and processed for Western blotting, as described in Methods. Mean±SD. n=3. *P<0.05 vs control. ##P<0.01 vs MPTP group.
Discussion
The present study indicated that administration of DHM significantly attenuates MPTP-induced behavioral impairments and DA neuron loss in an experimental mouse model of Parkinson's disease. Direct DA neuronal protection was further confirmed in a cultured DA neuronal cell line (MES23.5 cells). We found that, in addition to the inhibitory effect of DHM on ROS production, DHM administration inhibited GSK-3β activation in MES23.5 cells and in the striatum and SNc of mice. Moreover, DHM abolished MPP+-induced GSK-3β activation. Accordingly, inhibition of GSK-3β activity attenuated the MPTP-induced decrease in TH and VMAT2 expression. These data provide clear evidence that DHM elicits potent neuroprotective effects against MPTP lesions on DA neurons. In addition, our data demonstrate that DHM-mediated inhibition of GSK-3β may contribute to this protection.
DHM is a natural flavonoid extracted from an indigenous plant in southern China (Ampelopsis grossedentata). Previous reports have shown that DHM exhibits powerful anti-oxidative effects to protect cells from insult13,28. In addition, DHM has been shown to have potent anti-inflammatory effects on LPS-induced inflammation in RAW264.7 cells by inhibiting activation of the NF-κB and MAPK signaling pathways15. DHM was also shown to inhibit hepatocellular carcinoma growth through p5316,18. Recently, DHM was shown to improve behavioral deficits in a transgenic mouse model of Alzheimer's disease19. Moreover, one study reported that DHM may be a novel anti-alcohol intoxication medication20. In the present study, we showed that DHM pretreatment significantly attenuates MPTP-induced behavioral deficits and DA neuron impairments. We found that DHM directly protected DA neurons against MPP+ insults, which suggests that DHM may be a therapy for Parkinson's disease.
The GSK-3β pathway plays a critical role in the pathology of many neurodegenerative diseases, including Parkinson's disease. In addition, GSK-3β signaling is known to be involved in the regulation of neuronal apoptosis and neuroinflammation34,35,36,37. GSK-3β has become a treatment target for neurodegenerative and psychiatric diseases32,38. In the present study, we found that DHM stimulated phosphorylation of GSK-3β in DA cells in a time- and dose-dependent manner. DHM also attenuated MPP+-induced activation of GSK-3β in cultured DA cells and in MPTP-treated mice, which likely contributes to DHM-mediated DA neuronal protection. In addition to DHM-mediated suppression of ROS production, the present study provides the first evidence that DHM modulates the Akt/GSK-3β pathway, which may contribute to the neuroprotective effects of DHM. However, the precise mechanism underlying DHM-mediated regulation of the Akt/GSK-3β signaling pathway is unclear.
In summary, the present study showed that DHM elicits potent neuroprotective effects on DA neurons by modulating the Akt/GSK-3β pathway. The data reveal that DHM may be a promising candidate for Parkinson's disease treatment.
Author contribution
Xue-chu ZHEN and Ting CAO designed research; Zhao-xiang REN and Ya-fei ZHAO performed research; Zhao-xiang REN analyzed data; Ting CAO and Zhao-xiang REN wrote the papaer.
References
Kalia LV, Lang AE . Parkinson's disease. Lancet 2015; 386: 896–912.
Ye N, Neumeyer JL, Baldessarini RJ, Zhen X, Zhang A . Update 1 of recent progress in development of dopamine receptor subtype-selective agents: potential therapeutics for neurological and psychiatric disorders. Chem Rev 2013; 113: PR123–PR78.
Moon HE, Paek SH . Mitochondrial dysfunction in Parkinson's disease. Exp Neurol 2015; 24: 103–16.
Morris G, Berk M . The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med 2015; 13: 68.
Segura-Aguilar J, Kostrzewa RM . Neurotoxin mechanisms and processes relevant to Parkinson's disease: An Update. Neurotox Res 2015; 27: 328–54.
Vera Dias EJ . The role of oxidative stress in Parkinson's disease. J Parkinson Dis 2013; 3: 461–91.
Camilleri A, Vassallo N . The centrality of mitochondria in the pathogenesis and treatment of Parkinson's disease. CNS Neurosci Ther 2014; 20: 591–602.
Politis M, Niccolini F . Serotonin in Parkinson's disease. Behav Brain Res 2015; 277: 136–45.
Zhao R, Lu W, Fang X, Guo L, Yang Z, Ye N, et al. (6aR)-11-Amino-N-propyl-noraporphine, a new dopamine D2 and serotonin 5-HT1A dual agonist, elicits potent antiparkinsonian action and attenuates levodopa-induced dyskinesia in a 6-OHDA-lesioned rat model of Parkinson's disease. Pharmacol Biochem Be 2014; 124: 204–10.
Pinna A, Morelli M . A critical evaluation of behavioral rodent models of motor impairment used for screening of antiparkinsonian activity: The case of adenosine A(2A) receptor antagonists. Neurotox Res 2014; 25: 392–401.
Zheng J, Yang Z, Li X, Li L, Ma H, Wang M, et al. Optimization of 6-heterocyclic-2-(1H-pyrazol-1-yl)-N-(pyridin-2-yl)pyrimidin-4-amine as potent adenosine A2A receptor antagonists for the treatment of Parkinson's disease. ACS Chem Neurosci 2014; 5: 674–82.
Zhang H, Ye N, Zhou S, Guo L, Zheng L, Liu Z, et al. Identification of N-propylnoraporphin-11-yl 5-(1,2-dithiolan-3-yl)pentanoate as a new anti-Parkinson's agent possessing a dopamine D2 and serotonin 5-HT1A dual-agonist profile. J Med Chem 2011; 54: 4324–38.
Jiang B, Le L, Pan H, Hu K, Xu L, Xiao P . Dihydromyricetin ameliorates the oxidative stress response induced by methylglyoxal via the AMPK/GLUT4 signaling pathway in PC12 cells. Brain Res Bull 2014; 109: 117–26.
Liao W, Ning Z, Ma L, Yin X, Wei Q, Yuan E, et al. Recrystallization of dihydromyricetin from Ampelopsis grossedentata and its anti-oxidant activity evaluation. Rejuv Res 2014; 17: 422–9.
Hou XL, Tong Q, Wang WQ, Shi CY, Xiong W, Chen J, et al. Suppression of inflammatory responses by dihydromyricetin, a flavonoid from Ampelopsis grossedentata, via inhibiting the activation of NF-kappaB and MAPK signaling pathways. J Nat Prod 2015; 78: 1689–96.
Jiang L, Zhang Q, Ren H, Ma S, Lu C, Liu B, et al. Dihydromyricetin enhances the chemo-sensitivity of nedaplatin via regulation of the p53/Bcl-2 pathway in hepatocellular carcinoma cells. PLos One 2015; 10: e0124994.
Liu B, Tan X, Liang J, Wu S, Liu J, Zhang Q, et al. A reduction in reactive oxygen species contributes to dihydromyricetin-induced apoptosis in human hepatocellular carcinoma cells. Sci Rep 2014; 4: 7041.
Zhang Q, Liu J, Liu B, Xia J, Chen N, Chen X, et al. Dihydromyricetin promotes hepatocellular carcinoma regression via a p53 activation-dependent mechanism. Sci Rep 2014; 4: 4628.
Liang J, Lopez-Valdes HE, Martinez-Coria H, Lindemeyer AK, Shen Y, Shao XM, et al. Dihydromyricetin ameliorates behavioral deficits and reverses neuropathology of transgenic mouse models of Alzheimer's disease. Neurochem Res 2014; 39: 1171–81.
Shen Y, Lindemeyer AK, Gonzalez C, Shao XM, Spigelman I, Olsen RW, et al. Dihydromyricetin as a novel anti-alcohol intoxication medication. J Neurosci 2012; 32: 390–401.
Shi L, Zhang T, Liang X, Hu Q, Huang J, Zhou Y, et al. Dihydromyricetin improves skeletal muscle insulin resistance by inducing autophagy via the AMPK signaling pathway. Mol Cell Endocrinol 2015; 409: 92–102.
Zhou T, Zu G, Zhang X, Wang X, Li S, Gong X, et al. Neuroprotective effects of ginsenoside Rg1 through the Wnt/beta-catenin signaling pathway in both in vivo and in vitro models of Parkinson's disease. Neuropharmacology 2016; 101: 480–9.
Liu W, Li Y, Jalewa J, Saunders-Wood T, Li L, Holscher C . Neuroprotective effects of an oxyntomodulin analogue in the MPTP mouse model of Parkinson's disease. Eur J Pharmacol 2015; 765: 284–90.
Hong J, Sha S, Zhou L, Wang C, Yin J, Chen L . Sigma-1 receptor deficiency reduces MPTP-induced parkinsonism and death of dopaminergic neurons. Cell Death Dis 2015; 6: e1832.
Viana SD, Fernandes RC, Canas PM, Silva AM, Carvalho F, Ali SF, et al. Presymptomatic MPTP mice show neurotrophic S100B/mRAGE striatal levels. CNS Neurosci Ther 2016; 22: 396–403.
Wang D, Wong HK, Feng YB, Zhang ZJ . Paeoniflorin, a natural neuroprotective agent, modulates multiple anti-apoptotic and pro-apoptotic pathways in differentiated PC12 cells. Cell Mol Neurobiol 2013; 33: 521–9.
Velasco G, Kumar D, Das B, Sen R, Kundu P, Manna A, et al. Andrographolide analogue induces apoptosis and autophagy mediated cell death in U937 cells by inhibition of PI3K/Akt/mTOR pathway. PLoS One 2015; 10: e0139657.
Hou X, Tong Q, Wang W, Xiong W, Shi C, Fang J . Dihydromyricetin protects endothelial cells from hydrogen peroxide-induced oxidative stress damage by regulating mitochondrial pathways. Life Sci 2015; 130: 38–46.
Zhang Y, Liu S, Wang L, Wu Y, Hao J, Wang Z, et al. A novel PI3K/AKT signaling axis mediates Nectin-4-induced gallbladder cancer cell proliferation, metastasis and tumor growth. Cancer Lett 2016; 375: 179–89.
Li J, Song Z, Wang Y, Yin Y, Liu Y, Yuan R, et al. Overexpression of SphK1 enhances cell proliferation and invasion in triple-negative breast cancer via the PI3K/AKT signaling pathway. Tumour Biol 2016. Doi: 10.1007/s13277-016-4954-9.
Babichev Y, Kabaroff L, Datti A, Uehling D, Isaac M, Al-Awar R, et al. PI3K/AKT/mTOR inhibition in combination with doxorubicin is an effective therapy for leiomyosarcoma. J Transl Med 2016; 14: 67.
Zou Y, Wang R, Guo H, Dong M . Phytoestrogen beta-ecdysterone protects PC12 cells against MPP+-induced neurotoxicity in vitro: Involvement of PI3K-Nrf2-regulated pathway. Toxicol Sci 2015; 147: 28–38.
Liu Y, Zhang RY, Zhao J, Dong Z, Feng DY, Wu R, et al. Ginsenoside Rd protects SH-SY5Y cells against 1-methyl-4-phenylpyridinium induced injury. Int J Mol Sci 2015; 16: 14395–408.
Golpich M, Amini E, Hemmati F, Ibrahim NM, Rahmani B, Mohamed Z, et al. Glycogen synthase kinase-3 beta (GSK-3beta) signaling: implications for Parkinson's disease. Pharmacol Res 2015; 97: 16–26.
Cole AR . Glycogen synthase kinase 3 substrates in mood disorders and schizophrenia. FEBS J 2013; 280: 5213–27.
Morales-García JA, Susín C, Alonso-Gil S, Pérez DI, Palomo V, Pérez C, et al. Glycogen synthase kinase-3 inhibitors as potent therapeutic agents for the treatment of Parkinson disease. ACS Chem Neurosci 2013; 4: 350–60.
Yu Y, Wang JR, Sun PH, Guo Y, Zhang ZJ, Jin GZ, et al. Neuroprotective effects of atypical D1 receptor agonist SKF83959 are mediated via D1 receptor-dependent inhibition of glycogen synthase kinase-3 beta and a receptor-independent anti-oxidative action. J Neurochem 2008; 104: 946–56.
Wu Y, Shang Y, Sun S, Liang H, Liu R . Erythropoietin prevents PC12 cells from 1-methyl-4-phenylpyridinium ion-induced apoptosis via the Akt/GSK-3beta/caspase-3 mediated signaling pathway. Apoptosis 2007; 12: 1365–75.
Acknowledgements
This work was supported by funds from the National Natural Science Foundation of China (No 81130023 and 30825042) and the National Basic Research Plan (973) of the Ministry of Science and Technology of China (2011CB504403). The study was also supported by the Priority Academic Program Development of Jiangsu Higher Education Institutes (PAPD) and a Jiangsu key laboratory grant (BM2013003).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ren, Zx., Zhao, Yf., Cao, T. et al. Dihydromyricetin protects neurons in an MPTP-induced model of Parkinson's disease by suppressing glycogen synthase kinase-3 beta activity. Acta Pharmacol Sin 37, 1315–1324 (2016). https://doi.org/10.1038/aps.2016.42
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.42
Keywords
This article is cited by
-
Pazopanib alleviates neuroinflammation and protects dopaminergic neurons in LPS-stimulated mouse model by inhibiting MEK4-JNK-AP-1 pathway
Acta Pharmacologica Sinica (2023)
-
Dihydromyricetin Protects Against Salsolinol-Induced Toxicity in Dopaminergic Cell Line: Implication for Parkinson’s Disease
Neurotoxicity Research (2023)
-
Dysregulation of iron homeostasis and methamphetamine reward behaviors in Clk1-deficient mice
Acta Pharmacologica Sinica (2022)
-
Dihydromyricetin Protects Against Ethanol-Induced Toxicity in SH-SY5Y Cell Line: Role of GABAA Receptor
Neurotoxicity Research (2022)
-
Molecular Mechanism of Platelet-Derived Growth Factor (PDGF)-BB-Mediated Protection Against MPP+ Toxicity in SH-SY5Y Cells
Journal of Molecular Neuroscience (2021)