Abstract
Aim:
Cinnamon extracts rich in procyanidin oligomers have shown to improve pancreatic β-cell function in diabetic db/db mice. The aim of this study was to identify the active compounds in extracts from two species of cinnamon responsible for the pancreatic β-cell protection in vitro.
Methods:
Cinnamon extracts were prepared from Cinnamomum tamala (CT-E) and Cinnamomum cassia (CC-E). Six compounds procyanidin B2 (cpd1), (−)-epicatechin (cpd2), cinnamtannin B1 (cpd3), procyanidin C1 (cpd4), parameritannin A1 (cpd5) and cinnamtannin D1 (cpd6) were isolated from the extracts. INS-1 pancreatic β-cells were exposed to palmitic acid (PA) or H2O2 to induce lipotoxicity and oxidative stress. Cell viability and apoptosis as well as ROS levels were assessed. Glucose-stimulated insulin secretion was examined in PA-treated β-cells and murine islets.
Results:
CT-E, CC-E as well as the compounds, except cpd5, did not cause cytotoxicity in the β-cells up to the maximum dosage using in this experiment. CT-E and CC-E (12.5–50 μg/mL) dose-dependently increased cell viability in both PA- and H2O2-treated β-cells, and decreased ROS accumulation in H2O2-treated β-cells. CT-E caused more prominent β-cell protection than CC-E. Furthermore, CT-E (25 and 50 μg/mL) dose-dependently increased glucose-stimulated insulin secretion in PA-treated β-cells and murine islets, but CC-E had little effect. Among the 6 compounds, trimer procyanidins cpd3, cpd4 and cpd6 (12.5–50 μmol/L) dose-dependently increased the cell viability and decreased ROS accumulation in H2O2-treated β-cells. The trimer procyanidins also increased glucose-stimulated insulin secretion in PA-treated β-cells.
Conclusion:
Trimer procyanidins in the cinnamon extracts contribute to the pancreatic β-cell protection, thus to the anti-diabetic activity.
Similar content being viewed by others
Introduction
As a common spice, cinnamon may be a potential treatment for type 2 diabetes. Several clinical studies found that cinnamon supplements decreased the fasting serum glucose, triglyceride, total cholesterol and HbA1c levels in type 2 diabetic patients1,2,3. Furthermore, cinnamon extracts improved the glucose profiles in animal models. Qin et al found that cinnamon extract enhanced the insulin sensitivity in normal rats4. The blood glucose level in streptozotocin-induced diabetic rats or type 2 diabetic db/db mice was also reduced after cinnamon administration5,6,7,8. Although these studies suggest a beneficial effect of cinnamon in treating diabetes, the biological effects of cinnamon in the treatment of type 2 diabetes remain controversial. For example, some studies reported that cinnamon supplementation did not have any significant effects on type 2 diabetic patients9,10.
Cinnamon is produced from the bark of multiple species of Cinnamomum, which contains a variety of compounds, such as cinnamaldehyde, eugenol, and polyphenols11. Recently, active water-soluble components were isolated and found to mediate beneficial effects on glucose metabolism12,13,14. These components were identified as polyphenols, also named procyanidins, which usually occur as oligomers of epicatechin and the flavonoid catechin13. Our previous studies also confirmed that procyanidin oligomer-rich cinnamon extracts improved the glucose profiles in diabetic animals7,8. In a further study on the hypoglycemic effect of cinnamon extracts on db/db mice, we found that extracts isolated from two species of the genus Cinnamomum, Cinnamomum cassia (C cassia) and Cinnamomum tamala (C tamala), both exerted different anti-diabetic pharmacological effects, which improved not only the insulin sensitivity but also the pancreatic β-cell function in db/db mice15. Therefore, we hypothesized that β-cell protection is an important mechanism for the anti-diabetic effect of cinnamon. Furthermore, our other study confirmed that a one trimer procyanidin oligomer isolated from cinnamon extract, cinnamtannin D-1, protected pancreatic β-cells from lipotoxicity16.
Therefore, to explore the mechanisms involved in the anti-diabetic effect of cinnamon, further investigations of β-cell protection using cinnamon extracts or purified procyanidin oligomers are important. The objective of this study was to verify, in vitro, the protective effects of cinnamon extracts from C cassia and C tamala on lipotoxic pancreatic β-cells, as well as the effects of different procyanidin oligomers isolated from these two species of Cinnamomum.
Materials and methods
Plants and sample preparation
C cassia and C tamala were collected in 2014 from the Guangxi and Yunnan provinces, respectively. The samples were botanically authenticated by Professor Guan-yun GU at the School of Pharmacy, Fudan University. The voucher specimens, numbers RG012 and RG013, were deposited at the Herbarium of the Department of TCM Chemistry, School of Pharmacy of Shanghai University of Traditional Chinese Medicine (Shanghai, China). The extraction methods for the two cinnamon samples, C cassia and C tamala, and the analysis of these two cinnamon extracts and six compounds isolated from the extracts were described in our previous study15,17. The bioactivity of CC-E (bark extract from C cassia), CT-E (bark extract from C tamala) and six compounds was investigated in this study. The high performance liquid chromatography (HPLC) chromatographic profiles of CC-E and CT-E are provided in the supplementary data, which was described in our previous study15,17. The HPLC fingerprints and structures of the compounds, procyanidin B2 (cpd1), (−)-epicatechin (cpd2), cinnamtannin B1 (cpd3), procyanidin C1 (cpd4), parameritannin A1 (cpd5) and cinnamtannin D1 (cpd6), are shown in Figure 1. These compounds were analyzed by HPLC using a solvent system with 0.1% acetic acid and acetonitrile, and the purity of the compounds was greater than 95% (Supplementary Table 3 and 4).
Identification of CC-E and CT-E. (A) RP-HPLC chromatographic profile of CC-E. (B) RP-HPLC chromatographic profile of CT-E. (C) The structures of procyanidin B2 (compound 1, cpd1), (−)-epicatechin (compound 2, cpd2), cinnamtannin B1 (compound 3, cpd3), procyanidin C1 (compound 4, cpd4), parameritannin A1 (compound 5, cpd5) and cinnamtannin D1 (compound 6, cpd6). The purity of the compounds was >95%.
Cell culture and isolation of murine islets
All reagents used in cell culture were purchased from GIBCO (Carlsbad, CA, USA). The INS-1 rat insulinoma cell line was cultured in Roswell Park Memorial Institute (RPMI)-1640 medium. The MIN6 mouse insulinoma cell line was cultured in Dulbecco's modified Eagle medium (DMEM) containing 25 mmol/L glucose18. A concentration of 10% fetal bovine serum (FBS) and 50 μmol/L of β-mercaptoethanol were added to the medium to maintain cell growth. Murine islets were isolated from 8-week old male C57BL6 mice, described in our previous study16. All animal experiments were permitted by the IACUC of Shanghai Institute of Materia Medica. All cells were kept at 37 °C and 5% CO2 in humidified air.
Cell viability assay, Hoechst 33342 staining and reactive oxygen species (ROS) determination
Cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma-Aldrich, St Louis, MO, USA) assay as previously described16. To detect the cytotoxicity of each compound, different doses of CC-E, CT-E and the six procyanidins were added into the medium and incubated for 48 h separately. To determine the effect of the compounds on cell viability in palmitic acid (PA) or H2O2 treated pancreatic β-cells, INS-1 and MIN6 cells were treated with 0.4 mmol/L PA in the presence of different doses of compounds for 48 h. For the H2O2 model, the cells were pre-incubated with compounds for 4 h and subsequently exposed to 0.5 mmol/L H2O2 with compounds for another 2 h. Then, cell viability was measured using the MTT assay. The value was calculated as the inhibition ratio (% of control group).
Hoechst 33342 staining was performed to observe the effect of CC-E and CT-E on PA-induced apoptosis in INS-1 cells. The cells were incubated with 0.4 mmol/L PA in the presence of different doses of extracts for 48 h. Then, Hoechst 33342 staining was performed as previously described16. The apoptotic ratio in each group was calculated as the apoptotic cell number divided by the total cell number.
For ROS determination, the cells were treated with different doses of CC-E, CT-E or procyanidins for 4 h. Then, the ROS level was measured as previously described16. The fluorescence of the intracellular ROS was observed using fluorescence microscopy (DP70, Olympus, Tokyo, Japan), and the fluorescence absorption of ROS was also measured using a microplate reader (FlexStation III, Molecular device, Sunnyvale, CA, USA).
Glucose stimulated insulin secretion (GSIS)
The cells/islets were seeded into 24-well plates (100 000 cells or 20 islets per well). Then, the cells/islets were incubated with 0.4 mmol/L PA in the presence/absence of different doses of CC-E or CT-E for 48 h. The GSIS assay was performed as previously described16.
Immunofluorescence
The translocation of PDX1 into the nucleus was detected by immunofluorescence. Cultured islets were incubated with 0.4 mmol/L PA in the presence/absence of 25 or 50 μg/mL of CT-E for 48 h. Then, the islets were fixed in 4% paraformaldehyde for 2 h and washed in PBS. After incubation in 0.1% Triton-X100 at 37 °C for 30 min, the islets were incubated with rabbit anti-PDX1 antibody (1:100, Upstate, New York, NY, US) at 4 °C overnight. After multiple washes, AlexaFluor488-conjugated secondary antibody was incubated with the islets for 2 h at room temperature to label the PDX1 with green fluorescence. Cell nuclei were stained with Hoechst 33342 dye.
Statistical analysis
All data are expressed as the mean±SD. One-way ANOVA followed by Dunnett's test was used to calculate differences, and P<0.05 was considered statistically significant.
Results
CC-E and CT-E protected INS-1 β-cells from PA-induced dysfunction and apoptosis
As shown in Figure 2A, there was no obvious cytotoxicity of CC-E or CT-E on INS-1 pancreatic β-cells up to 100 μg/mL. However, the PA-induced decrease in cell viability was suppressed by both CC-E and CT-E in a dose-dependent manner (Figure 2A). Interestingly, CT-E had a better ameliorative effect on PA-induced cells than CC-E. To further determine whether the alteration of cell viability was due to apoptosis, Hoechst 33342 staining was performed to detect the apoptotic ratio in PA treated INS-1 cells in the presence/absence of CC-E or CT-E. The nuclei of the apoptotic cells displayed highly condensed chromatin upon Hoechst 33342 staining compared to normal cells. In PA-treated INS-1 cells, the apoptotic ratio was 6–7-fold greater than the control (Figure 2B and 2C). However, co-incubation with both CC-E and CT-E dose-dependently reduced the PA-induced apoptosis, and CT-E showed better protective effects (Figure 2B and 2C).
CC-E and CT-E protected INS-1 cells from PA-induced apoptosis. (A) After INS-1 cells were incubated with/without 0.4 mmol/L PA in the presence/absence of different concentrations of CC-E and CT-E for 48 h, the cell viability was measured using an MTT assay. Mean±SD. n=6. *P<0.05, **P<0.01 vs PA treated alone group. (B) After different treatments for 48 h, Hoechst 33342 staining was performed to observe apoptotic cells. Cell nuclei were stained with blue fluorescence, the apoptotic nuclei showed increased fluorescence. Scale bar=100 μm and applies to all panels. (C) The apoptotic rate was calculated as the apoptotic cell number divided by the total cell number. Mean±SD. n=10. *P<0.05, **P<0.01 vs PA treated alone group.
CC-E and CT-E attenuated H2O2-induced dysfunction and increase in ROS level in INS-1 β-cells
The effect of cinnamon extracts on acute H2O2-induced oxidative stress and apoptosis in INS-1 β-cells was tested. Figure 3A shows that pre-incubation with CC-E and CT-E dose-dependently suppressed the H2O2-induced decrease in cell viability. Additionally, both the intracellular ROS determination and fluorescent staining confirmed that H2O2-stimulated ROS generation was inhibited by these two cinnamon extracts (Figure 3B and 3C).
CC-E and CT-E attenuated H2O2-induced decrease in cell viability and ROS generation. (A) After INS-1 cells were pre-incubated with different concentrations of CC-E or CT-E for 4 h, 0.5 mmol/L H2O2 was added to the culture medium in the presence of compounds for another 2 h. Then, cell viability was measured using an MTT assay. Mean±SD. n=6. *P<0.05, **P<0.01 vs H2O2-treated alone group. (B) The ROS probe was pre-incubated with INS-1 cells in the presence of CC-E or CT-E, and after H2O2 stimulation, the fluorescence optical density was measured to evaluate ROS generation. Mean±SD. n=6. *P<0.05, **P<0.01 vs H2O2-treated alone group. (C) The intracellular ROS was imaged using fluorescence microscopy. The presence of ROS is indicated by green fluorescence. Scale bar=100 μm and applies to all panels.
The protective effect of procyanidin oligomers on INS-1 β-cells
The effect of the six main procyanidin oligomers isolated from CC-E and CT-E on PA-impaired INS-1 cells was investigated. Except for cpd5 (tetramer procyanidin parameritannin A1) at 50 and 100 μmol/L concentrations, there was no cytotoxicity of these compounds on INS-1 cells up to 100 μmol/L (Figure 4A and 4B). We found that cpd3 and cpd6 dose-dependently protected INS-1 cells from the PA-induced decrease in cell viability (Figure 4A and 4B). Furthermore, cpd4, the B-type trimer procyanidin C1, generated weaker protective effect on cells (Figure 4B). It was also confirmed that cpd3, cpd4 and cpd6 inhibited the H2O2-induced decrease in cell viability and H2O2-stimulated ROS generation in a dose-dependent manner (Figure 4C and 4D). However, there was a less protective effect of cpd1 or cpd2 on PA-treated INS-1 cells.
Procyanidin oligomers protected INS-1 cells from PA- or H2O2-induced apoptosis. (A) After INS-1 cells were incubated with/without 0.4 mmol/L PA in the presence/absence of different concentrations of cpd1–3 for 48 h, the cell viability was measured using an MTT assay. Mean±SD. n=6. **P<0.01 vs PA treated alone group. (B) After treatment for 48 h (cpd4–6), the cell viability was measured using an MTT assay. Mean±SD. n=6. **P<0.01 vs PA treated alone group. (C) After treatment with cpd3, cpd4 or cpd6 and H2O2, the cell viability was measured using an MTT assay. Mean±SD. n=6. *P<0.05, **P<0.01 vs H2O2 treated alone group. (D) After treatment with cpd3, cpd4 or cpd6 and H2O2, the ROS generation was measured. Mean±SD. n=6. *P<0.05, **P<0.01 vs H2O2 treated alone group.
CT-E and trimer procyanidins improved insulin secretion in lipotoxic β-cells
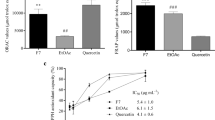
We next investigated the effect of CC-E and CT-E on insulin secretion in INS-1 cells and primary cultured murine islets. After incubation with 0.4 mmol/L PA in the presence/absence of different doses of CC-E or CT-E for 48 h, INS-1 cells were subsequently stimulated with 11.1 mmol/L glucose for 1 h, and the secreted insulin was then detected. The insulin secretion in PA-treated INS-1 cells was decreased compared to control cells (Figure 5A). Co-treatment with CT-E dose-dependently recovered insulin secretion in PA-treated cells (Figure 5A). However, another cinnamon extract, CC-E, affected GSIS in lipotoxic β-cells less (Figure 5A). We also tested the effect of procyanidins on insulin secretion in β-cells. The three trimer procyanidins, cpd3, cpd4 and cpd6 25 μmol/L, also improved insulin secretion in lipotoxic β-cells (Figure 5B). However, the other procyanidins, cpd1, cpd2 and cpd5, had less effect (Figure 5B).
Cinnamon extracts and procyanidin oligomers improved GSIS in lipotoxic β-cells. (A) After INS-1 cells were treated with PA in the presence/absence of CC-E and CT-E for 48 h, 11.1 mmol/L glucose-stimulated insulin secretion was analyzed. Mean±SD. n=3. *P<0.05, **P<0.01 vs PA-treated alone group. (B) After INS-1 cells were treated with PA in the presence/absence of 25 μmol/L procyanidins for 48 h, 11.1 mmol/L GSIS was analyzed. *P<0.05, **P<0.01 vs PA treated alone group. n=3. (C) After murine islets were treated with PA in the presence/absence of different doses of CT-E for 48 h, 5 mmol/L and subsequently 25 mmol/L glucose-stimulated insulin secretion assays were performed. Mean±SD. n=3. *P<0.05, **P<0.01 vs PA-treated alone group. (D) After murine islets were treated with PA in the presence/absence of different doses of CT-E for 48 h, the islets were stimulated with 25 mmol/L glucose for 1 h, then PDX1 immunofluorescence was analyzed. Green fluorescence shows PDX1 staining, and blue fluorescence indicates the nucleus in islets. Representative images are presented, scale bar=100 μm and applies to all panels.
We further studied the protective effect of CT-E on GSIS in PA-treated murine islets. Similar to the results in INS-1 β-cells, CT-E also protected cultured islets from the PA-induced decrease in GSIS (Figure 5C). Chronic treatment with PA also reduced the translocation of PDX1 into the nucleus (Figure 5D), thereby inhibiting insulin secretion. During co-treatment with CT-E, PDX1 translocation was partly restored in lipotoxic islets (Figure 5D), suggesting a protective effect of CT-E on pancreatic β-cells.
Discussion
Although multiple studies have shown that cinnamon extract improves glucose profiles, the protective effect of cinnamon on pancreatic β-cells has not been well studied. Only Bisht et al proposed that cinnamon extracts could protect pancreatic β-cells and improve insulin secretion, but no direct evidence was provided19. To further investigate the detailed mechanism, we studied the effect of the two extracts on pancreatic β-cells using pancreatic β-cell lines and primary cultured islets. PA is the most common saturated fatty acid in animals, and it mediates acute and chronic effects on pancreatic β-cells20. Long-term exposure to PA results in increased β-cell dysfunction and apoptosis21. By contrast, inhibition of PA-induced β-cell apoptosis using small molecule compounds or natural products may serve as potential therapies for type 2 diabetes22. Therefore, the effect of CC-E and CT-E on PA-impaired pancreatic β-cells was investigated using pancreatic β-cell lines and cultured islets. We verified that both CC-E and CT-E protected the rat insulinoma cell line INS-1. Similar results were found in mouse insulinoma cell line MIN6 (Supplementary Figure 2) pancreatic β-cell lines from PA-induced apoptosis. Furthermore, CC-E and CT-E both improved insulin secretion in PA-treated INS-1 β-cells and cultured islets. Moreover, although complicated mechanisms were involved in PA-induced β-cell dysfunction, using an H2O2 model, we confirmed that the protective effect of CC-E and CT-E on β-cells was at least partially by reducing ROS-induced injury.
Taken together, our in vivo15 and in vitro studies confirm the beneficial effect of CC-E and CT-E on pancreatic β-cells. However, similar to many other nutraceuticals, the precise biological effects of cinnamon extracts on type 2 diabetes are unclear. Notably, the source or genus of plants used in previous studies was not always clarified. Additionally, the precise identification of their constituents was also unknown. Moreover, combinations using various anti-diabetic drugs with cinnamon also contributed to these controversial results. Therefore, we identified the main oligomeric procyanidins in the extracts of two cinnamon species, C cassia and C tamala. The main procyanidins from CC-E and CT-E were different as follows: CC-E mainly contains procyanidin B2 (cpd1), procyanidin C1 (cpd4), and less (−)-epicatechin (cpd2) and cinnamtannin B1 (cpd3), whereas CT-E mainly contains cinnamtannin B1 (cpd3), cinnamtannin D1 (cpd6) and less parameritannin A1 (cpd5). This may explain why CT-E had better protective effects on pancreatic β-cells than CC-E both in vivo and in vitro because the different procyanidin constituents in these two cinnamon extracts contributed to their diverse pharmacological effects.
In this study, we reported the protective effect of different procyanidin oligomers on pancreatic β-cells for the first time. It was found that cpd3 and cpd6, A-type trimer procyanidin oligomers, protected against PA-induced dysfunction. Reducing ROS production may be a possible mechanism involved in the protective effect of these procyanidins. Although not as effective as cpd3 or cpd6, the B-type trimer procyanidin cpd4 also had a protective effect on β-cells. By contrast, cpd5 was toxic to INS-1 cells, whereas cpd1 and cpd2 had no protective effect on pancreatic β-cells. The trimer procyanidins, cinnamtannin B1 and D1, inhibit cyclooxygenase-223, whereas cinnamtannin B1 exerts anti-oxidative effects in pancreatic acinar cells24. Our recent study also showed that cinnamtannin D1 protects against PA-induced β-cell dysfunction by attenuating oxidative stress and reducing nuclear factor kappa B (NF-κB) activation16. Therefore, the trimer procyanidin oligomers are important components of cinnamon that protect β-cells, in which A-type trimers are more effective. Furthermore, cpd3 and cpd6 are the main components in CT-E, whereas CC-E only contained cpd4 and a minor amount of cpd315. This may be why the protective effect of CT-E was superior to CC-E on pancreatic β-cells both in vitro and in vivo.
Given the above, pancreatic β-cell protection contributes to the anti-diabetic bioactivity of cinnamon extracts. Furthermore, trimer procyanidins, particularly A-type procyanidin oligomers, may be the main active components in cinnamon extracts. This study verified the effect of different procyanidin oligomers on lipotoxic pancreatic β-cells for the first time, which provides a better understanding of the pharmacological mechanisms of cinnamon extracts in the treatment of diabetes.
Author contribution
He-yao WANG and Yi-ming LI designed the research and revised the manuscript. Peng SUN and Ting WANG performed the research. Lu CHEN isolated compounds. Peng SUN analyzed data and wrote the manuscript. Bang-wei YU performed part of the cell experiments. Hui-min FAN, Qi JIA, and Kai-xian CHEN contributed to the research design.
References
Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA . Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 2003; 26: 3215–8.
Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth DO, et al. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest 2006; 36: 340–4.
Crawford P . Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: a randomized, controlled trial. J Am Board Fam Med 2009; 22: 507–12.
Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y . Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res Clin Pract 2003; 62: 139–48.
Kim SH, Hyun SH, Choung SY . Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J Ethnopharmacol 2006; 104: 119–23.
Li R, Liang T, Xu L, Li Y, Zhang S, Duan X . Protective effect of cinnamon polyphenols against STZ-diabetic mice fed high-sugar, high-fat diet and its underlying mechanism. Food Chem Toxicol 2013; 51: 419–25.
Lu Z, Jia Q, Wang R, Wu X, Wu Y, Huang C, et al. Hypoglycemic activities of A- and B-type procyanidin oligomer-rich extracts from different Cinnamon barks. Phytomedicine 2011; 18: 298–302.
Jia Q, Liu X, Wu X, Wang R, Hu X, Li Y, et al. Hypoglycemic activity of a polyphenolic oligomer-rich extract of Cinnamomum parthenoxylon bark in normal and streptozotocin-induced diabetic rats. Phytomedicine 2009; 16: 744–50.
Vanschoonbeek K, Thomassen BJ, Senden JM, Wodzig WK, van Loon LJ . Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J Nutr 2006; 136: 977–80.
Blevins SM, Leyva MJ, Brown J, Wright J, Scofield RH, Aston CE . Effect of cinnamon on glucose and lipid levels in non insulin-dependent type 2 diabetes. Diabetes Care 2007; 30: 2236–7.
Rafehi H, Ververis K, Karagiannis TC . Controversies surrounding the clinical potential of cinnamon for the management of diabetes. Diabetes Obes Metab 2012; 14: 493–9.
Anderson RA, Broadhurst CL, Polansky MM, Schmidt WF, Khan A, Flanagan VP, et al. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J Agric Food Chem 2004; 52: 65–70.
Anderson RA . Chromium and polyphenols from cinnamon improve insulin sensitivity. Proc Nutr Soc 2008; 67: 48–53.
Cheng DM, Kuhn P, Poulev A, Rojo LE, Lila MA, Raskin I . In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem 2012; 135: 2994–3002.
Chen L, Sun P, Wang T, Chen K, Jia Q, Wang H, et al. Diverse mechanisms of antidiabetic effects of the different procyanidin oligomer types of two different cinnamon species on db/db mice. J Agric Food Chem 2012; 60: 9144–50.
Wang T, Sun P, Chen L, Huang Q, Chen K, Jia Q, et al. Cinnamtannin D-1 protects pancreatic beta-cells from palmitic acid-induced apoptosis by attenuating oxidative stress. J Agric Food Chem 2014; 62: 5038–45.
Chen L, Yang Y, Yuan P, Yang Y, Chen K, Jia Q, et al. Immunosuppressive effects of A-type procyanidin oligomers from Cinnamomum tamala. Evid Based Complement Alternat Med 2014; 2014: 365258.
Minami K, Yano H, Miki T, Nagashima K, Wang CZ, Tanaka H, et al. Insulin secretion and differential gene expression in glucose-responsive and -unresponsive MIN6 sublines. Am J Physiol Endocrinol Metab 2000; 279: E773–81.
Bisht S, Sisodia SS . Assessment of antidiabetic potential of Cinnamomum tamala leaves extract in streptozotocin induced diabetic rats. Indian J Pharmacol 2011; 43: 582–5.
Giacca A, Xiao C, Oprescu AI, Carpentier AC, Lewis GF . Lipid-induced pancreatic beta-cell dysfunction: focus on in vivo studies. Am J Physiol Endocrinol Metab 2011; 300: E255–62.
Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M . Fatty acid signaling in the beta-cell and insulin secretion. Diabetes 2006; 55 Suppl 2: S16–23.
Sun P, Wang T, Zhou Y, Liu H, Jiang H, Zhu W, et al. DC260126: a small-molecule antagonist of GPR40 that protects against pancreatic beta-cells dysfunction in db/db mice. PLoS One 2013; 8: e 66744.
Killday KB, Davey MH, Glinski JA, Duan P, Veluri R, Proni G, et al. Bioactive A-type proanthocyanidins from Cinnamomum cassia. J Nat Prod 2011; 74: 1833–41.
Rivera-Barreno R, del Castillo-Vaquero A, Salido GM, Gonzalez A . Effect of cinnamtannin B-1 on cholecystokinin-8-evoked responses in mouse pancreatic acinar cells. Clin Exp Pharmacol Physiol 2010; 37: 980–8.
Acknowledgements
We thank Prof S SEINO for his gift of MIN6 cells. This work was supported by a grant from the National Natural Science Foundation (No 81473262 and 21172044), the National Science and Technology Major Project (Key New Drug Creation and Manufacturing Program, 2012ZX09301001-001 and 2013ZX09103001-001), the Natural Science Foundation of Shanghai, China (No 15ZR1441200) and Shanghai Three-year Plan on Promoting TCM Development (No ZY3-LCPT-2-1003).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary information is available on the website of Acta Pharmacologica Sinica.
Supplementary information
Supplementary Figure S1
Identification of CC-E and CT-E. (TIF 248 kb)
Supplementary Figure S2
CC-E, CT-E and procyanidin oligomers protected MIN6 cells from PA-induced dysfunction and apoptosis. (TIF 811 kb)
Supplementary Table S1
Identification of major peaks in total ion chromatogram of CC-E (DOC 28 kb)
Supplementary Table S2
Identification of major peaks in total ion chromatogram of CT-E (DOC 30 kb)
Supplementary Table S3
The purity of cpd1 to 6. (DOC 31 kb)
Supplementary Table S4
HPLC chromatogram of cpd1 to 6. (DOC 161 kb)
Rights and permissions
About this article
Cite this article
Sun, P., Wang, T., Chen, L. et al. Trimer procyanidin oligomers contribute to the protective effects of cinnamon extracts on pancreatic β-cells in vitro. Acta Pharmacol Sin 37, 1083–1090 (2016). https://doi.org/10.1038/aps.2016.29
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.29
Keywords
This article is cited by
-
The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice
Nature Metabolism (2021)