Abstract
Aim:
B cell-activating factor belonging to the TNF family (BAFF) is a member of TNF family and required for peripheral B cell survival and homeostasis. BAFF has been shown to promote the proliferation of T and B cells. In this study we examined whether and how BAFF mediated the interaction between mouse T and B cells in vitro.
Methods:
BAFF-stimulated B or T cells were co-cultured with T or B cells. The interactions between T and B cells were analyzed by measuring the expression of co-stimulatory molecules (CD28/CD80 or CD40/CD154), the proliferation and secretion of T and B cells and other factors. Two siRNAs against the transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) and BAFF receptor (BAFF-R) were used to identify the receptors responsible for the actions of BAFF.
Results:
BAFF-stimulated B cells significantly promoted the proliferation and activity of co-cultured T cells, and increased the percentages of CD4+CD28+ and CD4+CD154+ T cells. Similarly, BAFF-stimulated T cells significantly promoted the proliferation and activity of co-cultured B cells, and increased CD19+CD80+ and CD19+CD40+B cell subpopulations. BAFF-R siRNA-silenced B cells showed significantly lower expression of CD40 and CD80 than the control B cells. When the BAFF-R siRNA-silenced B cells were stimulated with BAFF, then co-cultured with T cells, the expression of CD28 and CD154 on T cells was not increased. TACI siRNA-silenced B cells exhibited higher expression of CD40 and CD80 than the control B cells. When the TACI siRNA-silenced B cells were stimulated with BAFF, then co-cultured with T cells, the expression of CD28 and CD154 on T cells was significantly increased.
Conclusion:
BAFF upregulates CD28/B7 and CD40/CD154 expression, and promotes the interactions between T and B cells in a BAFF-R-dependent manner.
Similar content being viewed by others
Introduction
B lymphocytes play an important role as antigen-presenting cells (APCs) that facilitate the activation of T cells1. The interaction between T and B lymphocytes is required for antigen responses and antibody production. Two signal systems mediate this interaction. The first signal system involves type II major histocompatibility complex (MHC II), antigen peptide and T cell receptors (TCRs). The second signal system involves co-stimulatory molecules, including CD28/B7 (CD80, CD86) and CD40/CD154 (CD40 ligand)2,3. The CD28/B7 interaction is mainly involved in the activation and proliferation of T lymphocytes, whereas the CD40/CD40L interaction is mainly involved in B lymphocyte activation and proliferation. Electron microscopy has shown that T and B lymphocytes are spatially close to each other, which not only facilitates the interactions of surface molecules on cells (eg, TCR-MHC, CD28/B7, CD40/CD40L, and other adhesive molecules) but also promotes the efficient secretion of cytokines4. The proliferation of B lymphocytes is modulated by the cytokines secreted from T lymphocytes, whereas the activation of T lymphocytes relies on the antigen-presenting function of B lymphocytes5.
B cell-activating factor belonging to the TNF family (BAFF) is required for peripheral B cell survival and homeostasis6. Our previous results have shown that the proliferation of T and B lymphocytes is enhanced by stimulation with BAFF. BAFF promoted the production of CD4+CD154+ and CD4+CD69+ T lymphocytes and CD19+CD21+, CD19+CD23+, and CD19+/IgD+ B lymphocytes7. BAFF plays a vital role in the development of autoimmune disorders. High serum levels of BAFF have been clinically demonstrated in RA patients8.
There are currently three identified receptors of BAFF: B cell maturation antigen (BCMA), transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), and BAFF receptor (BAFF-R)9. TACI is expressed in both B lymphocytes and activated T lymphocytes. TACI has emerged as an unusual TNF receptor-like molecule with a sophisticated mode of action. However, the functions of TACI have not been fully elucidated. TACI−/− mice have revealed two functions of this receptor: upregulation of T cell-independent immune responses and downregulation of B cell activation and expansion10. BAFF-R is highly expressed in resting B cells and is also detectable in resting CD4+ T cells. BAFF-R is crucial for the survival of mature B cells and homeostasis in the peripheral lymphoid organs. BAFF/BAFF-R signals mediate PI3K/Akt/mTOR signaling, which is involved in antibody production by B lymphocytes from collagen-induced arthritis rats8. BAFF/BAFF-R signaling also induces the expression of CD21 and CD23 on the B cell surface, promoting the survival of the mature B cell. FO and MZ cells will die in the absence of either BAFF-R or BAFF11. By contrast, BCMA appears to be a B cell-specific BAFF receptor. BAFF's function in B cell proliferation and activation is mediated through these three receptors12,13.
These findings raise some questions. Is BAFF involved in the interaction between T and B lymphocytes? If so, does BAFF mediate these interactions by regulating the expression of co-stimulatory molecules, such as CD28/B7 and CD40/CD154? Which receptor is the main receptor through which BAFF mediates the interaction between T and B lymphocytes? The answers to these questions are still unclear. The current study aimed to investigate the mechanisms by which BAFF mediates the interaction between T and B lymphocytes in mice and to determine whether TACI or BAFF-R plays a significant role. The results of this study showed that BAFF upregulated the co-stimulatory molecules CD28/B7 and CD40/CD154 and promoted the interactions between T and B lymphocytes. TACI-Ig and anti-BAFF antibody inhibited the interactions between T and B lymphocytes by neutralizing BAFF and repressing the expression of co-stimulatory molecules. BAFF-R rather than TACI was the receptor through which BAFF promotes the interaction of T and B lymphocytes.
Materials and methods
Reagents, antibodies and primers
Anti-mouse PE-CD28, PE-CD154, PE-CD40, PE-CD80, FITC-CD19, and FITC-CD4 antibodies were purchased from eBioscience, Inc (San Diego City, California, USA). The primers were synthesized by Sangon Biotech (Shanghai, China). TRIzol and Platinum SYBR Green were purchased from Invitrogen Co (Beijing, China). Enzyme-linked immunosorbent assay (ELISA) kits for IL-2, IL-17, IgM, and IgG were purchased from Research & Development (R&D, USA). Anti-CD28, anti-CD154, anti-CD80, and anti-CD40 antibodies were purchased from Biosynthesis Co (Beijing, China). Transwell inserts (0.4 μm) were from Corning Co, Ltd (USA). TACI-Ig was a kind gift from Dr WX WANG (Rongchang Pharmaceutical Research Institute, Beijing, China). The anti-mouse BAFF antibody (AF2106) was from R&D, and the BLyS (310-13) was from PeproTech Co, Ltd (USA).
Mice
DBA/1 mice (male, 18±2 g) were obtained from the Shanghai Institute of Materia Medica, Chinese Academy of Sciences (production license No: SCXK [HU] 2008-0016). All mice were maintained in a specific-pathogen-free animal facility at Anhui Medical University. All experiments were approved by the Ethics Review Committee for Animal Experimentation of the Institute of Clinical Pharmacology at Anhui Medical University.
B cell magnetic separation
B cells were isolated from mouse spleens by positive selection using magnetic cell separation (MACS). For this purpose, a single cell suspension from mouse spleen (approximately 1×108 cells) in 500 μL of MACS buffer was incubated with PE-anti-CD19 for 20 min, followed by incubation with anti-PE beads for an additional 20 min. The cell suspension was loaded onto an LS column (Miltenyi Biotec, German). After washing with degassed buffer three times, labeled B cells were collected.
Proliferation assays14
T and B lymphocyte proliferation was analyzed using [3H]TdR intake (Institute of the Atomic Energy, Beijing, China). The murine thymus and spleen were removed under sterile conditions. T or B lymphocyte suspensions (200 μL) in DMEM supplemented with 10% fetal calf serum were seeded in 96-well culture plates and stimulated by BAFF (1 μg/mL). Cells were plated in triplicates and cultured for 48 h. Six hours before the end of the culture period, 20 μL of [3H]TdR was added to each well. Radioactivity was measured using the LS6500 liquid scintillation counter (Beckman Co, USA). The results were described as the average of counts per minute measured in triplicate.
Secretion assays
T or B cell suspensions (500 μL) were seeded into 24-well culture plates and stimulated by BAFF (1 μg/mL). After 48 h, T and B lymphocyte supernatants were collected and analyzed for IL-2 and IL-17 (from T lymphocyte culture supernatants) or IgG and IgM (from B lymphocytes) by ELISA.
T and B cell co-culture system
T and B lymphocytes were collected as described above. T or B lymphocytes (5×106/well) were cultured and stimulated by BAFF (PeproTech, 1 μg/mL). At the same time, anti-BAFF (0.1 μg/mL) and TACI-Ig (10 μg/mL) were used in the control groups. The cultures were incubated at 37 °C in an incubator (Shellab Co, USA) with 5% CO2 for 6 h. B or T lymphocytes were washed to remove BAFF and then collected and co-cultured with T or B lymphocytes in a 12-transwell culture plate. The ratio of B cells to T cells was 1:1. Experiments were performed in triplicate. After 24 h of co-culture, supernatants were collected for analysis of IL-2 and IL-17 or IgM. T or B lymphocytes were collected to determine the proportions of CD4+CD154+ and CD4+CD28+ T lymphocytes or CD19+CD40+ and CD19+CD80+ B lymphocytes. The protein expressions of CD154 and CD28 or CD40 and CD80 were also analyzed.
Analysis of IL-2, IL-17, IgG, and IgM levels in culture supernatants
Levels of IL-2, IL-17, IgG, and IgM in cell culture supernatants were determined using ELISA kits (R&D Systems, Shanghai, China). All assays were performed according to the manufacturer's instructions. Optical densities were measured at 450 nm with an ELISA plate reader (ELx808, BioTek, USA).
Flow cytometry analysis15
The cells were cultured as described above. T or B cells in transwell inserts were then collected. A 100 μL aliquot of thymus or spleen lymphocyte suspension was then transferred to a 12×75 mm polystyrene round-bottom tube. The antibody combinations CD28-PE/CD4-FITC (10 μL, each 5 μL) or CD154-PE/CD4-FITC (10 μL, each 5 μL) were added into round-bottom tubes for T lymphocytes. Similarly, CD40-PE/CD19-FITC (10 μL, each 5 μL) or CD80-PE/CD19-FITC (10 μL, each 5 μL) antibody combinations of 10 μL were added to each tube for B lymphocytes. These samples were gently mixed and incubated for 20 min at 4°C. Cell-associated fluorescence was analyzed using a FACScan instrument (Epics XL, Beckman Coulter, USA) and Cell Quest software (Beckman Coulter, USA).
Measurement of CD28, CD154, CD80, and CD40 protein levels16
Cells were cultured and collected as described above. Cells were lysed in RIPA lysis buffer (50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 10 mmol/L PMSF, 1 mmol/L EDTA, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate) for 20 to 30 min on ice. Protein concentration was determined with the Lowry protein assay. The proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). Protein-containing membranes were then incubated with primary antibodies (anti-CD28, anti-CD154, anti-CD80, and anti-CD40) overnight at 4 °C. Immunoblots were incubated with the appropriate horseradish peroxidase conjugated goat anti-mouse IgG (at 1:30 000/1:40 000 dilutions). The intensity of each band was quantified with Bio-Rad Quantity One software (Bio-Rad, Hercules, CA, USA). All experiments reported here were performed in triplicate and the results were reproducible.
Cell transfection
The design and synthesis of TACI siRNAs and BAFF-R siRNAs were performed by Qiagen Co, Ltd (Shanghai, China). The nucleotide sequences of sense and antisense TACI siRNA are as follows:
TACI siRNA, SI02717631 5′-CCCTGTATGCGTGCAACAGTA-3′, SI02742523 5′-CCCAGCCTGTGGAAACGTGTA-3′, BAFF-R siRNA, SI00235062 5′-ATGGTGTAGGATTGAGGCTAA-3′, SI00235074 5′-CACCTCCACAGACTTCTGCAA-3′.
One nucleofection sample contained 2×106 cells, 10 mL of 20 mmol/L siRNA and 100 mL Nucleofector Solution V (Amaxa, Germany). Transfection was performed by mixing the nucleofection sample with 10 mL siRNA, and the samples was then transferred to an Amaxa-certified cuvette. The sample was transferred into 12-well plates after completion. After 10 h, TACI or BAFF-R siRNA-silenced B cells were collected to measure the expressions of TACI or BAFF-R mRNA and the mRNA expression of CD40 and CD80.
T cell and BTACI- cell co-culture system
TACI or BAFF-R siRNA-silenced B cells (5×106/well) were stimulated by BAFF (1 μg/mL) and cultured in DMEM for 6 h. B cells were then co-cultured with T cells in a 12-transwell culture plate. The ratio of the two cell types was 1:1. After 24 h, T cells were collected, and the mRNA expression of CD28 and CD154 was analyzed by quantitative real-time PCR (qPCR).
qPCR analysis of mRNA expression of TACI, BAFF-R, CD40, and CD80 in BTACI- cells and CD28 and CD154 mRNA in T cells
Total RNA was extracted using TRIzol reagent according to the manufacturer's instructions. For PCR reactions, 10 μL of SYBR Green was added to 2 μL cDNA with 50 nmol/L primers in a 20 μL reaction, and the PCR conditions were 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Melt curve analysis was performed from 60°C to 95 °C. Aseptic distilled water was used to replace cDNA as a negative control. The primers for TACI, BAFF-R, CD40, CD80, CD28, CD154, and GAPDH were designed by Sangon Biotech Co, Ltd (Shanghai, China) and are listed in Table 1. Both target and normalizing gene PCR efficiencies were first determined to ensure that the normalizing genes were acceptable and to test primer efficiency. qPCR was carried out with a 2-fold dilution series from a pooled set of cDNA, and the threshold Ct value was plotted against the log cDNA dilution. Expression changes were calculated using the 2−ΔΔCt method and expressed as fold change relative to the control17.
Statistical analysis
All data are expressed as the mean±standard deviation and were analyzed by the SPSS (version 11.5) statistical package. The analysis was performed with an unpaired two-tailed Student's t-test. A P value of ≤0.05 was considered statistically significant and is indicated by a single asterisk.
Results
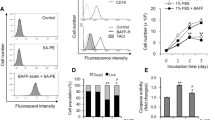
BAFF enhanced T and B lymphocyte proliferation and secretion
T or B cells were seeded into culture plates and stimulated by BAFF (1 μg/mL), and the proliferation of T and B cells was analyzed by [3H]TdR intake. The results showed that T and B lymphocyte proliferation increased with BAFF stimulation (Figure 1A). TACI-Ig and anti-BAFF treatment decreased the proliferation of T and B lymphocytes (Figure 1A). Culture supernatants were collected after 48 h, and the levels of IL-2, IL-17, IgG, and IgM were assessed. The levels of IL-2 and IL-17 were markedly higher in the BAFF group than in the untreated group. IgG and IgM levels also increased in the BAFF group. The levels of IL-2, IL-17, IgG, and IgM were reduced in both the TACI-Ig- and anti-BAFF-treated groups (Figure 1B, 1C).
BAFF promotes T and B lymphocyte proliferation and secretory functions. T and B lymphocyte proliferation was analyzed by [3H]TdR intake. The levels of IL-2, IL-17, IgG, and IgM were analyzed by ELISA. (A) Proliferation of T and B lymphocytes; (B) The levels of IL-2 and IL-17; (C) IgG and IgM are shown as the mean±SD of four samples pooled from three independent experiments, each performed in technical triplicates. *P<0.05, **P<0.01 vs T or B cells. #P<0.05, ##P<0.01 vs T or B cells+BAFF.
B lymphocytes activated by BAFF promote T lymphocyte function
A co-culture system was utilized to observe the effects of B lymphocytes activated by BAFF on T lymphocyte function. B lymphocytes were treated with BAFF (1 μg/mL), anti-BAFF or TACI-Ig. Six hours later, B lymphocytes were collected and co-cultured with T lymphocytes (1:1 ratio). After 24 h of co-culture, the levels of IL-2 and IL-17 in co-culture supernatants were significantly elevated in the group stimulated by BAFF relative to those of the control group (Figure 2A). Meanwhile, TACI-Ig significantly decreased IL-2 and IL-17 levels (Figure 2A). In the presence of BAFF, the proportions of CD4+CD154+ and CD4+CD28+ T lymphocytes were significantly higher than the control group. Compared with the BAFF stimulation group, both the TACI-Ig and anti-BAFF treatments decreased the proportions of the lymphocyte subsets mentioned above (Figure 2B, 2C). Additionally, the protein expression of CD154 and CD28 was increased in the BAFF group compared with the untreated group. Furthermore, TACI-Ig and anti-BAFF were shown to decrease the expression of CD154 and CD28 (Figure 2D, 2E). These results suggest that B lymphocytes activated by BAFF increase T lymphocyte functions by promoting the expression of CD154 and CD28.
B lymphocytes activated by BAFF promote T lymphocyte function. (A) ELISA of IL-2 and IL-17 levels. (B) Flow cytometry analysis of the proportions of CD4+CD154+ and CD4+CD28+ T lymphocytes. (C) Comparison of the proportions of CD4+CD154+ and CD4+CD28+ T lymphocytes in different treatment groups. (D) Western blot of the protein expression of CD154 and CD28. (E) Comparison of the protein expression of CD154 and CD28 in different treatment groups. Data are shown as the mean±SD of four samples pooled from three independent experiments, each performed in technical triplicates. *P<0.05, **P<0.01 vs T/B cells. #P<0.05, ##P<0.01 vs T/B cells+BAFF.
T lymphocytes activated by BAFF could promote B lymphocyte function
T lymphocytes were cultured and stimulated by BAFF and then co-cultured with B lymphocytes (1 μg/mL). After 24 h, co-culture supernatants were collected to measure IgM levels. B lymphocytes were also collected to determine the percentages of CD19+CD40+ and CD19+CD80+ B lymphocytes and the expression of CD40 and CD80. The results showed that the level of IgM in the B/T lymphocyte co-culture group was not significantly different from that of the B lymphocyte group. However, IgM levels in the B/T lymphocyte co-culture supernatant markedly increased in the presence of BAFF (Figure 3A). The concentration of IgM in the TACI-Ig treatment group decreased significantly (Figure 3A). In the presence of BAFF, the proportions of CD19+CD40+ and CD19+CD80+ B lymphocytes were higher than those without BAFF treatment (Figure 3B, 3C). Compared with the BAFF-treated group, both TACI-Ig and anti-BAFF treatments decreased the percentages of CD19+CD40+ and CD19+CD80+ B lymphocytes (Figure 3B, 3C). Compared with the untreated group, the protein expression of CD40 and CD80 increased in the BAFF-treated group (Figure 3D, 3E). TACI-Ig and anti-BAFF inhibited the increased protein expression of CD40 and CD80 (Figure 3D, 3E). These results suggest that T lymphocytes activated by BAFF could promote B lymphocyte function by inducing the expression of CD40 and CD80.
T lymphocytes activated by BAFF promote B lymphocyte proliferation and activity. (A) ELISA of IgM levels. (B) Flow cytometry analysis of the proportions of CD19+CD40+ and CD19+CD80+ B lymphocytes. (C) Comparison of the proportions of CD19+CD40+ and CD19+CD80+ B lymphocytes in different treatment groups. (D) Western blot of the protein expression of CD40 and CD80. (E) Comparison of the protein expression of CD40 and CD80 in different treatment groups. Data are shown as the mean±SD of four samples pooled from three independent experiments, each performed in technical triplicates. *P<0.05, **P<0.01 vs B/T cells. #P<0.05, ##P<0.01 vs B/T cells+BAFF.
BAFF-R rather than TACI might be the receptor through which BAFF promotes the interaction of T and B lymphocytes
The results above showed that BAFF could affect the interaction between T and B lymphocytes by promoting the expression of the co-stimulatory molecules CD28/B7 and CD40/CD40L. To determine which receptor, such as TACI and BAFF-R, was involved in the interaction between T and B lymphocytes mediated by BAFF, TACI siRNAs and BAFF-R siRNAs were transfected into B lymphocytes to obtain siRNA-silenced B cells. The mRNA expression of CD40 and CD80 in siRNA-silenced B cells was analyzed by qPCR. The results showed that the mRNA expression of CD40 and CD80 in TACI siRNA-silenced B cells was higher than normal B lymphocytes. Moreover, BAFF significantly promoted the expression of CD40 and CD80 mRNA (Figure 4A). The expression of CD40 and CD80 in BAFF-R siRNA-silenced B cells was lower than control B cells. Additionally, BAFF did not significantly promote the expression of CD40 and CD80 mRNA (Figure 4B).
Comparison of the mRNA expression of CD40 and CD80 in siRNA-silenced B cells and the effects of siRNA-silenced B cells on CD28 mRNA and CD154 mRNA expression in T cells. (A) The mRNA expression of CD40 and CD80 in TACI siRNA-silenced B cells was higher than normal B lymphocytes. Moreover, BAFF significantly promoted the expression of CD40 and CD80 mRNA (*P<0.01 vs B cell group. ##P<0.01 vs B cell+BAFF group. $$P<0.01 vs BTACI- cells). (B) The mRNA expression of CD40 and CD80 in BAFF-R siRNA-silenced B cells was lower than normal B lymphocytes. BAFF did not significantly promote the expression of CD40 and CD80 mRNA (*P<0.05, **P<0.01 vs B cell group. ##P<0.01 vs B cell+BAFF group). (C) The mRNA expression of CD28 and CD154 increased in the T/TACI siRNA-silenced B cell co-culture group. Moreover, BAFF significantly promoted the expression of CD28 and CD154 mRNA (*P<0.05, **P<0.01 vs T/B cell group. #P<0.05, ##P<0.01 vs T/B cell+BAFF group. $$P<0.01 vs T/BTACI- cells). (D) The expression of CD28 and CD154 in the T/BAFF-R siRNA-silenced B cell group was lower than the control co-culture group. BAFF did not significantly promote the expression of CD28 and CD154 mRNA (*P<0.05, **P<0.01 vs T/B cell group. ##P<0.01 vs T/B cell+BAFF group. $$P<0.01 vs T/BTACI- cells). Data are shown as the mean±SD of four samples pooled from three independent experiments, each performed in technical triplicates.
TACI or BAFF-R siRNA-silenced B cells (5×106/well) were stimulated by BAFF (1 μg/mL) and co-cultured with T cells (ratio 1:1). After 24 h, the mRNA expression of CD28 and CD154 was analyzed. Compared with the control co-culture group, the mRNA expression of CD28 and CD154 increased in the T/TACI siRNA-silenced B cell co-culture group. Moreover, BAFF significantly promoted the expression of CD28 and CD154 mRNA (Figure 4C). The expression of CD28 and CD154 in the T/BAFF-R siRNA-silenced B cell group was lower than the control co-culture group, and BAFF did not significantly promote the expression of CD28 and CD154 mRNA (Figure 4D).
Discussion
The interactions between T lymphocytes and B lymphocytes are required for B lymphocyte response to antigen and antibody production. These interactions mainly occur through co-stimulatory molecules, such as CD28/B7 and CD40/CD40L18. The co-stimulatory signal is required for T cell activation. The two signal model is required for T cell activation. The first signal results from T cell recognition of antigens on the surface of APCs and is an 'off' signal, unless the T cell simultaneously receives a second, or 'co-stimulatory' signal from the same APC, in which case the T cell is activated19,20. CD28 expressed on the surface of T cells is involved in cytokine production and provides important signals that lower the threshold of T cell activation, augment the production of IL-2, and promote T cell survival. CD28 binds to CD80 or CD86 (expressed on the activated APC), leading to a dramatic upregulation in IL-2 expression. The interaction of CD28 with CD80 or CD86 is critical for the activation, optimization, and regulation of T cell function21. CD28/B7 interactions also play a role as key modulators of plasma cell function. Under homeostatic conditions, the CD28/B7 interaction mediates plasma cell secretion; however, during inflammation, this pathway is altered to increase antibody production in local plasma cells22,23. In this study, we found that B lymphocytes activated by BAFF promote the expression of CD28 in T lymphocytes. In turn, T lymphocytes activated by BAFF were found to promote CD80 expression and IgM production from B lymphocytes.
CD154, a member of the TNF family, is mainly expressed on the surface of activated CD4+ T cells and binds to CD40 on B cells to provide signals necessary for the initiation of the immune response. These signals are involved in B cell activation, differentiation, and the production of pathogenic autoantibodies. CD154 plays a global role in various systems but is especially important in the physiopathology of the vascular system24. De-methylation of DNA reactivates the CD154 gene on the silenced X chromosome in T cells, which then stimulate B cells to produce a large amount of autoantibodies. Overexpression of CD154 may lead to high levels of IgM production through interactions between T and B lymphocytes25. The engagement of CD40 and CD154 regulates a wide spectrum of molecular and cellular processes, including the initiation and progression of cellular and humoral adaptive immunity. Thus, it is highly involved in the pathophysiology of inflammatory diseases, such as autoimmune diseases, atherothrombosis, cancer, and respiratory diseases26. Previous results from our laboratory have shown that the CD4+CD154+ T lymphocyte subset is larger following stimulation by BAFF. This study has shown that in a T/B lymphocyte co-culture system, T lymphocytes activated by BAFF promote CD80 expression and IgM production in B lymphocytes.
BAFF plays a very important role in B cell survival and differentiation, regulating the size and repertoire of the B cell subsets. It has thus been implicated as a potential driver of B cell hyperplasia and autoantibody production in human autoimmune diseases27. BAFF is increased in autoimmune disease and is correlated with disease activity. Elevated levels of BAFF have also been detected in the serum and synovial fluid of RA patients28. In this study, we first found that the proliferation and activity of T and B lymphocytes were increased with BAFF stimulation. This result suggests that BAFF is involved in the interactions between T and B lymphocytes. Furthermore, the levels of IL-2, IL-17, and IgM were significantly elevated in the supernatant of co-cultured T and B lymphocytes from normal mice after stimulation with BAFF. B lymphocytes activated by BAFF could promote T lymphocyte proliferation; conversely, T lymphocytes activated by BAFF could promote B lymphocyte proliferation. These results indicate that BAFF may promote the interactions between T and B lymphocytes.
We next determined whether the interactions between T and B cells regulated by BAFF were related to the CD28/B7 and CD40/CD154 co-stimulatory molecules. To explore this hypothesis, the effects of BAFF, anti-BAFF, and TACI-Ig on the expression of CD28, CD154, CD40, and CD80 were analyzed. Our results showed that B lymphocytes stimulated by BAFF increased the proportions of CD4+CD28+ and CD4+CD154+T lymphocyte subpopulations. In a reciprocal effect, T lymphocytes stimulated by BAFF induced increases in the proportions of CD19+CD80+ and CD19+CD40+ B lymphocyte subpopulations. We also demonstrated that BAFF enhances the protein expression of CD28 and CD154 in T cells and the protein expression of CD40 and CD80 in B cells in co-culture. These results suggest that BAFF promotes T and B lymphocyte interactions by upregulating the expressions of CD28/B7 and CD40/CD154.
TACI is mainly expressed on B cells, especially activated B cells. However, early data suggested that plasmablasts (specifically those originating from ''innate'' B cells) also express TACI and receive TACI-dependent differentiation and survival signals in response to multimeric BAFF. The function of TACI has been difficult to characterize. TACI provides direct or indirect negative signals in most B cells, either by inhibitory signals or by decreasing circulating BAFF levels through undescribed mechanisms29,30. BAFF-R is necessary for the survival of mature B cells. In the absence of BAFF or BAFF-R, the generation of mature B cells would be abolished. BAFF protects B cells from atrophy and provides B cell survival signals in vivo and in vitro31. To further investigate the function of TACI and BAFF-R in the interaction between T and B lymphocytes mediated by BAFF, siRNAs that silence TACI and BAFF-R were transfected into B cells to obtain siRNA-silenced B cells. The regulatory effects of siRNA-silenced B cells on T lymphocytes were observed. We found that the expression of CD40 and CD80 in TACI siRNA-silenced B cells was higher than that of normal B lymphocytes. The expression of CD40 and CD80 in BAFF-R siRNA-silenced B cells was lower than control B cells. The expression of CD40 and CD80 mRNA in TACI siRNA-silenced B cells stimulated by BAFF significantly increased relative to cells without BAFF. In co-culture experiments, the expression of CD28 and CD154 by T lymphocytes in the T/TACI siRNA-silenced B cell co-culture group was higher than that in the T/B co-culture group. BAFF-R siRNA-silenced B cells decreased the expression of CD28 and CD154 in T lymphocytes also. This result suggests that BAFF-R rather than TACI is the receptor through which BAFF promotes the interaction of T and B lymphocytes.
In summary, BAFF appears to promote the interactions of T and B lymphocytes by regulating the expression of co-stimulatory molecules, such as CD28/B7 and CD40/CD154. BAFF-R may be the receptor through which BAFF promotes the interaction of T and B lymphocytes. TACI-Ig and anti-BAFF exerted inhibitory effects on T and B lymphocyte interactions, which may be related to the neutralization of BAFF and the inhibition of co-stimulatory molecule expression. These findings further clarify the role of BAFF in mediating T/B cell interactions in immune regulation and inflammatory autoimmune diseases.
Author contribution
Feng ZHANG and Shan-shan SONG performed research, analyzed data and wrote the paper. Jin-ling SHU, Ying LI, Yu-jing WU, Qing-tong WANG, Jing-yu CHEN, Yan CHANG, and Hua-xun WU contributed to the research. Ling-ling ZHANG and Wei WEI analyzed data and wrote the paper.
References
Townsend MJ, Monroe JG, Chan AC . B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev 2010; 237: 264–83.
Rizvi M, Pathak D, Freedman JE, Chakrabarti S . CD40-CD40 ligand interactions in oxidative stress, inflammation and vascular disease. Trends Mol Med 2008; 14: 530–8.
Yang Y, Ratts RB, Hussain RZ, Northrop SC, Ben LH, Lovett-Racke A, et al. CD28:B7 interaction is necessary for the protective effect of T cell vaccination in EAE. Eur J Immunol 2007; 37: 2032–42.
Saito T, Hashimoto-Tane A . Dynamic regulation of T cell activation and co-stimulation through TCR-microclusters. FEBS Lett 2010; 584: 4865–71.
Liu S, Kandeva T, Tchervenkov J . CD1d-mediated interaction between activated T cells and B cells is essential to B-cell proliferation and anti-alpha-Gal antibody production. Transplant Proc 2009; 41: 398–402.
Vincent F, Northcott M, Hoi A, Mackay F, Morand E . Association of serum B cell activating factor from the tumour necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL) with central nervous system and renal disease in systemic lupus erythematosus. Lupus 2013; 22: 873–84.
Wang QT, Ma YK, Huang B, Liu DD, Wei W . Effect of rhTACI-Ig fusion protein on antigen-specific T cell responses from keyhole limpet haemocyanin challenged mice. Mol Immunol 2011; 49: 380–6.
Li PP, Liu DD, Liu YJ, Song SS, Wang QT, Chang Y, et al. BAFF/BAFF-R involved in antibodies production of rats with collagen-induced arthritis via PI3K-Akt-mTOR signaling and the regulation of paeoniflorin. J Ethnopharmacol 2012; 141: 290–300.
Schweighoffer E, Vanes L, Nys J, Cantrell D, McCleary S, Smithers N, et al. The BAFF receptor transduces survival signals by co-opting the B cell receptor signaling pathway. Immunity 2013; 38: 475–88.
Mackay F, Schneider P . TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev 2008; 19: 263–76.
Zhang X, Park CS, Yoon SO, Li L, Hsu YM, Ambrose C, et al. BAFF supports human B cell differentiation in the lymphoid follicles through distinct receptors. Int Immunol 2005; 17: 779–88.
Coquery CM, Erickson LD . Regulatory roles of the tumor necrosis factor receptor BCMA. Crit Rev Immunol 2012; 32: 287–305.
Alexaki VI, Notas G, Pelekanou V, Kampa M, Valkanou M, Theodoropoulos P, et al. Adipocytes as immune cells: differential expression of TWEAK, BAFF, and APRIL and their receptors (Fn14, BAFF-R, TACI, and BCMA) at different stages of normal and pathological adipose tissue development. J Immunol 2009; 183: 5948–56.
Liu Y, Zhang L, Wu Y, Tong T, Zhao W, Li P, et al. Therapeutic effects of TACI-Ig on collagen-induced arthritis by regulating T and B lymphocytes function in DBA/1 mice. Eur J Pharmacol 2011; 654: 304–14.
Zhang LL, Li PP, Song SS, Liu YJ, Wang QT, Chang Y, et al. Comparative efficacy of TACI-Ig with TNF-alpha inhibitor and methotrexate in DBA/1 mice with collagen-induced arthritis. Eur J Pharmacol 2013; 708: 113–23.
Liu DD, Li PP, Song SS, Liu YJ, Wang QT, Chang Y, et al. Pro-apoptotic effect of epigallo-catechin-3-gallate on B lymphocytes through regulating BAFF/PI3K/Akt/mTOR signaling in rats with collagen-induced arthritis. Eur J Pharmacol 2012; 690: 214–25.
Schmittgen TD, Livak KJ . Analyzing real-time PCR data by the comparative C (T) method. Nat Protoc 2008; 3: 1101–8.
Shipkova M, Wieland E . Surface markers of lymphocyte activation and markers of cell proliferation. Clin Chim Acta 2012; 413: 1338–49.
Kim EY, Teh HS . Critical role of TNF receptor type-2 (p75) as a costimulator for IL-2 induction and T cell survival: a functional link to CD28. J Immunol 2004; 173: 4500–9
Fuchs EJ . Transplantation tolerance: from theory to clinic. Immunol Rev 2014; 258: 64–79.
Mesturini R, Gigliotti CL, Orilieri E, Cappellano G, Soluri MF, Boggio E, et al. Differential induction of IL-17, IL-10, and IL-9 in human T helper cells by B7h and B7.1. Cytokine 2013; Doi: 10.1016/j.cyto.2013.05.021.
Njau MN, Jacob J . The CD28/B7 pathway: a novel regulator of plasma cell function. Adv Exp Med Biol 2013; 785: 67–75.
Njau MN, Kim JH, Chappell CP, Ravindran R, Thomas L, Pulendran B, et al. CD28-B7 interaction modulates short- and long-lived plasma cell function. J Immunol 2012; 189: 2758–67.
Hassan GS, Merhi Y, Mourad W . CD40 ligand: a neo-inflammatory molecule in vascular diseases. Immunobiology 2012; 217: 521–32.
Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML . B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev 2010; 237: 205–25.
Kawabe T, Matsushima M, Hashimoto N, Imaizumi K, Hasegawa Y . CD40/CD40 ligand interactions in immune responses and pulmonary immunity. Nagoya J Med Sci 2011; 73: 69–78 .
Boneparth A, Davidson A . B-cell activating factor targeted therapy and lupus. Arthritis Res Ther 2012; 14 Suppl 4: S2.
Wei F, Chang Y, Wei W . The role of BAFF in the progression of rheumatoid arthritis. Cytokine 2015; 76: 537–44.
Yan M, Wang H, Chan B, Roose-Girma M, Erickson S, Baker T, et al. Activation and accumulation of B cells in TACI-deficient mice. Nat Immunol 2001; 2: 638–43.
Ng LG, Ng CH, Woehl B, Sutherland AP, Huo J, Xu S, et al. BAFF costimulation of Toll-like receptor-activated B-1 cells. Eur J Immunol 2006; 36: 1837–46.
Otipoby KL, Sasaki Y, Schmidt-Supprian M, Patke A, Gareus R, Pasparakis M, et al. BAFF activates Akt and Erk through BAFF-R in an IKK1-dependent manner in primary mouse B cells. Proc Natl Acad Sci U S A 2008; 105: 12435–8.
Acknowledgements
The authors deeply thank Hui FANG for his excellent assistance in nucleofection and JT GREENE from the Medical Center of Ohio State University for language editing. This work was financially supported by the National Natural Science Foundation of China (No 81473223, 31100640 and 81330081) and the China Postdoctoral Science Foundation (No 2013M540509).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, F., Song, Ss., Shu, Jl. et al. BAFF upregulates CD28/B7 and CD40/CD154 expression and promotes mouse T and B cell interaction in vitro via BAFF receptor. Acta Pharmacol Sin 37, 1101–1109 (2016). https://doi.org/10.1038/aps.2016.15
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.15