Abstract
Aim:
Sulforaphane (SFN), a natural dietary isothiocyanate, is found to exert beneficial effects for cardiovascular diseases. This study aimed to investigate the mechanisms underlying the protective effects of SFN in a model of myocardial hypoxia/reoxygenation (H/R) injury in vitro.
Methods:
Cultured neonatal rat cardiomyocytes pretreated with SFN were subjected to 3-h hypoxia followed by 3-h reoxygenation. Cell viability and apoptosis were detected. Caspase-3 activity and mitochondrial membrane potential (ΔΨm) was measured. The expression of ER stress-related apoptotic proteins were analyzed with Western blot analyses. Silent information regulator 1 (SIRT1) activity was determined with SIRT1 deacetylase fluorometric assay kit.
Results:
SFN (0.1–5 μmol/L) dose-dependently improved the viability of cardiomyocytes, diminished apoptotic cells and suppressed caspase-3 activity. Meanwhile, SFN significantly alleviated the damage of ΔΨm and decreased the expression of ER stress-related apoptosis proteins (GRP78, CHOP and caspase-12), elevating the expression of SIRT1 and Bcl-2/Bax ratio in the cardiomyocytes. Co-treatment of the cardiomyocytes with the SIRT1-specific inhibitor Ex-527 (1 μmol/L) blocked the SFN-induced cardioprotective effects.
Conclusion:
SFN prevents cardiomyocytes from H/R injury in vitro most likely via activating SIRT1 pathway and subsequently inhibiting the ER stress-dependent apoptosis.
Similar content being viewed by others
Introduction
Ischemic heart disease, including acute myocardial infarction (AMI), is a leading cause of morbidity and mortality worldwide1. Early reperfusion is the best strategy for the salvation of ischemic myocardium. However, myocardial reperfusion leads to some problems, such as excessive production of reactive oxygen species (ROS), Ca2+ overload, endoplasmic reticulum (ER) stress and the activiation of apoptotic pathways, all of which contribute to post-ischemic cardiomyocyte death and deteriorate myocardial injury2. Novel pharmacological or other effective therapy approaches to mitigate reperfusion injury are sorely needed.
Sulforaphane (SFN) is a dietary isothiocyanate which is mainly found in cruciferous vegetables3. It has been reported to possess extensive pharmacological effects, including anti-oxidative, anticancer and modulating inflammation properties3,4,5. Moreover, there have been additional studies implicating the cardioprotective role of SFN6. SFN has been proven to protect neonatal rat cardiomyocytes from oxidative stress in vitro7,8 and to blunt postischemic cardiac injury in vivo9. However, the mechanism involved in the cardioprotective role of SFN is still unclear.
Some studies indicated that hypoxia/reoxygenation (H/R) was associated with ER stress. It has been demonstrated that H/R injury could induce cardiomyocyte apoptosis by triggering ER stress10. The marker proteins of ER stress, including GRP78, CHOP and caspase-12 were elevated during H/R injury11. The silent information regulator 1 (SIRT1) signaling pathway plays an essential role in cell survival and cardioprotection against H/R injury12. It has been shown that SIRT1 is implicated in the changes of cardiomyocyte apoptosis and infarct size. Inhibition of SIRT1 by pharmacological or genetic interference elicited cardiac cell apoptosis and increased infarct size during I/R injury13,14. Both ER stress and SIRT1 signaling pathway are now recognized to play an essential function in deciding cardiac cell life and death. More recently, studies showed that a close relationship may exist between ER and SIRT115. However, little is known between them during I/R injury.
The aims of the present study were to determine the protective effects of SFN on cardiomyocytes exposed to H/R injury and to elucidate the mechanism of SFN protecting cardiomyocytes against ER stress-induced apoptosis by SIRT1 signaling pathway during H/R injury.
Materials and methods
Reagents
SFN was obtained from Sigma-Aldrich (St Louis, MO, USA) and diluted in 10% dimethyl sulfoxide (DMSO) (Cell Signaling Technology, Inc, Beverly, MA, USA). The final concentration of DMSO never exceeded 0.1% in either control or treated cells. Specific SIRT1 inhibitor Ex-527 was also obtained from Selleck Chemicals (Houston, USA). Fetal bovine serum and DMEM were purchased from Gibco Co Ltd (USA). The lactate dehydrogenase (LDH) commercial kit was obtained from Biovision (Mountain View, CA, USA). Bax (1:1000), Bcl-2 (1:500) and SIRT (1:1000) antibodies were purchased from Abcam (Cambridge, MA, USA). GRP78 (1:1000), cleaved caspase-12 (1:1000) and CHOP (1:1000) antibodies were purchased from Cell Signaling Technology Inc (Beverly, MA, USA).
Primary culture of neonatal rat cardiomyocytes and simulated ischemia reperfusion in vitro
Cardiomyocytes were isolated from 1 to 2-d-old Sprague-Dawley rats as previously described16. Sprague-Dawley rats were obtained from the Laboratory Animal Center of Guangdong Province (Guangzhou, China). All animals received humane care in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institute of Health (NIH Publication No 85-23, revised 1996). All investigations were approved by the Bioethics Committee of Southern Medical University, Guangzhou, China. The isolated cells were cultured in DMEM with 10% FBS and 1% penicillin/streptomycin, and then maintained in 5% CO2 incubator at 37 °C. After 72 h, cultured cardiomyocytes were used in subsequent experiments. To simulate the ischemia reperfusion model, cardiomyocytes were plated in a hypoxia chamber (5% CO2 and 95% N2) for 3 h at 37 °C, followed by reoxygenation in a normoxia chamber (5% CO2 and 95% O2) for 3 h at 37 °C in DMEM with 10% FBS.
The experimental design was divided into four steps
Step 1: After 72 h, cultured cardiomyocytes were randomly divided into five groups: 1) control group: cardiomyocytes were maintained in normoxic condition without any treatment; 2) H3/R3 group: Hypoxia 3 h, following Reoxygenation 3 h; 3) H3/R6 group: Hypoxia 3 h, following Reoxygenation 6 h; 4) H3/R9 group: Hypoxia 3 h, following Reoxygenation 9 h; 5) H3/R12 group: Hypoxia 3 h, following Reoxygenation 12 h.
Step 2: When the reoxygenation duration of experimental model was determined, cardiomyocytes were randomly divided into six groups to be exposed to the optional concentration: control group, H/R group and SFN groups (0.1, 0.5, 1 and 5 μmol/L).
Step 3: When ideal concentration of SFN was screened out, cardiomyocytes were randomly divided into four groups: 1) control group: cardiomyocytes were treated with PBS and incubated in normoxic condition. 2) SFN group: cardiomyocytes were treated with 5 μmol/L SFN and incubated in normoxic condition. 3) H/R group: as above design, cardiomyocytes were subjected to hypoxia for 3 h, followed by reoxygenation for 3 h. 4) SFN+H/R group: cardiomyocytes pretreated with 5 μmol/L SFN were exposed to 3 h of hypoxia followed by 3 h reoxygenation. SFN was pretreated 1 h before hypoxia.
Step 4: To explore the role of SIRT1 in myocardial protection with SFN pretreatment during H/R injury, cardiomyocytes were randomly divided into six groups (Figure 1): 1) Control group; 2) Ex-527 (E) group; 3) H/R group; 4) Ex-527 (E)+H/R group; 5) SFN+H/R group; 6) SFN (5 μmol/L)+Ex-527 (E)+H/R group. Ex-527 was pretreated 1 h before hypoxia. The concentration of Ex-527 is 1 μmol/L.
Experimental protocol of step 4. Control and Ex-527 (E) group: Cardiomyocytes were treated with PBS and E for 1 h respectively, then incubated in normoxic condition for 6 h. The four groups [H/R, H/R+E, sulforaphane (SFN)+H/R and SFN+E+H/R] were treated with PBS, E, SFN, SFN+E for 1 h respectively, then exposed to 3-h hypoxia followed by 3-h reoxygenation.
Cell viability analysis
Cell viability was determined by the MTS Cell Proliferation Assay kit as previously described17,18. Cells were seeded at a density of 4×104 cells/well in a flat-bottomed 96-well plates. After the treatment, 20 μL MTS solution was added to each well and incubated for 3 h at 37 °C. The absorbance measurement was detected at 490 nm and used to calculate the relative ratio of cell viability.
LDH measurement
Lactate dehydrogenase (LDH) leakage is usually monitored to evaluate the extent of cell injury. After experimental treatment, 100 μL of culture medium was taken to assess LDH levels by measuring the conversion of pyruvate to lactate. The activities of LDH was analyzed with microplates at 450 nm accoring to the manufacturer's instruction.
Detection of apoptotic cells
Evaluation of apoptosis was performed with a commercially available cell death detection kit to find DNA strand breaks using the terminal deoxynucleotidyl transferase-mediated dUDP nickend labeling (TUNEL) reagent according to the manufacturer's protocol (Promega). Cells that showed positive TUNEL staining in the nuclei were identified as apoptotic.To further visualize fragmented nuclei, Hoechst 33258 staining was used to identify the morphological features of apoptosis as previously described19. Cardiomyocytes were fixed with 4% paraformaldehyde (pH 7.4) for 1 h at room temperature and washed three times in PBS. Cells were then stained with Hoechst 33258 for 15 min and washed in PBS three times. Apoptotic cells with nuclear staining of Hoechst 33258 contained characters of apoptosis, condensed chromatin and nuclear shrinkage. The percentage of apoptosis cells were counted from each optical field as the apoptotic ratio in comparison to control group.
Determination of mitochondrial membrane potential (ΔΨm)
Mitochondrial membrane potential (MMP) was measured with a cationic dye of 5,5′,6,6′-tetrachloro 1,1′,3,3′-tetraethylbenzimidazolcarbocyaenina iodide (JC-1) as previously described20. Cardiomyocytes were incubated with fluorescent probe JC-1 for 30 min at 37 °C. After incubation, the cells were washed in PBS for three times. Fluorescence was imaged at a 488 nm excitation wavelength, and emission signals were captured at 590 and 530 nm for red and green channels with confocal microscopy, separately, and the JC-1 ratio always refers to the red/green ratio.
Measurement of caspase-3 activity
Caspase-3 activity was measured by using a commercialized caspase-3 assay kit (BioVision Inc, Milpitas Blvd, Calif, USA). Caspase-3 activity was expressed as optical density. The absorbance at 405 nm of the released pNA was monitored with a spectrophotometer. The relative activity of enzyme is evaluated through the rate of absorption value in treatment group and normal group. We suppose the activity in normal group is 1.
SIRT1 activity analysis
Sirt 1 activity was determined with SIRT1 Deacetylase Fluorometric Assay kit as previously described21. Nuclear proteins were extracted using the Nuclear and Cytoplasmic Protein Extraction kit according to the manufacturer's instructions. The resulting fluorescence was measured at 340 nm excitation and 440 nm emission wavelengths with a fluorescent microplate reader.
Western blotting analysis
Cardiomyocytes were washed by ice PBS for three times. Cell lysates were centrifuged at 12 000 r/min at 4 °C for 15 min, and the supernatant was collected. The samples were denatured by SDS sample buffer and in boiling water for 10 min, exposed to 10% SDS-PAGE, and then electroblotted onto nitrocellulose membranes. The membranes were blocked by 5% non-fat milk for 2 h at room temperature. The membranes were incubated overnight at 4 °C with different primary antibodies. The membranes were rinsed with TBS-T and incubated with the secondary HRP-conjugated antibodies. GAPDH antibody was used as the loading control. The data analysis were used with Image-Pro Plus through measuring the densities of immunoreactive bands.
Statistical analysis
All of the values were expressed as the mean±SD and statistically ananlyzed with SPSS 19.0. One-way ANOVA was performed to test significance of biochemical data of different groups. A difference of P<0.05 was considered to be statistically significant. For each assessment, at least three independent experiments were performed.
Results
SFN alleviated H/R injury
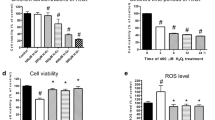
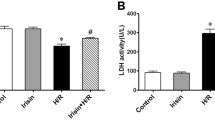
To explore the optional reoxygenation time, cell viability of different time durations was measured by MTS. As shown in Figure 2A, cell viability significantly declined in a time-dependent manner (P<0.05). The release of LDH was used as an index of cardiomyocyte injury. LDH activity increased with prolonged duration of reoxygenation (Figure 2B). Taken together, to conduct our experiment with a relatively ideal cell viability and to provide an accurate result, we decided the optional duration for mimicking I/R is hypoxia 3 h and reoxygenation 3 h.
Sulforaphane (SFN) protects against H/R-induced injury of primary neonatal rat cardiomyocytes. (A) Cardiomyocytes were pretreated with SFN followed H/R and cell viability was determined by MTS. (B) The release of LDH in culture medium after H/R was determined. (C) The toxic effect of different concentrations of SFN (0–5 μmol/L) was measured in primary neonatal rat cardiomyocytes by MTS. (D) The effect of SFN on H/R-induced injury in primary neonatal rat cardiomyocytes. Data are expressed as Mean±SD. n=3. bP<0.05 vs control; eP<0.05 vs H/R.
We next determined the effect of SFN on cradiomyocytes viability during H/R. The results demonstrated that H/R caused a significant decline in cell viability compared with the control group (P<0.05). Pretreatment with SFN (0.1, 0.5, 1, and 5 μmol/L) was found to increase cell viability significantly in a dose-dependent manner. The cell viability went to the peak at a concentration of 5 μmol/L (P<0.05 vs H/R group, Figure 2C and 2D). Therefore, 5 μmol/L SFN was used in treated group for subsequent experiments.
SFN attenuated H/R-elicited apoptotis in cultured neonatal rat cardiomyocytes
As shown in Figure 3, representative photomicrographs of TUNEL assay results depicted apoptotis of H/R-induced cardiomyocytes. TUNEL staining showed that the apoptotic cells increased remarkedly in the I/R group compared with the control group and SFN decreased apoptotic cells obviously (P<0.05, Figure 3A and 3B) Caspase-3 has been known as a pivotal protein in the final pathway of apoptosis22. To further characterize the inhibitory effect of SFN on myocardial cell apoptosis, we examined whether SFN could suppress caspase-3 activity. In our present study, the activity of caspase-3 was significantly greater in H/R group than that in control group (P<0.05). However, SFN could decrease the activity of caspase-3 which was induced by H/R injury (P<0.05, Figure 3C). Therefore, SFN could attenuate H/R-induced apoptosis in cultured neonatal rat cardiomyocytes.
Effect of Sulforaphane (SFN) on the apoptosis of cardiomyocytes after H/R injury. (A) Under fluorescence microscope (×200). Green dots indicate the typical positive cells. Blue dots indicate cell nucleus. (B) Apoptotic index in cardiomyocytes. (C) Caspase-3 activity in cardiomyocytes. Data are expressed as Mean±SD of three independent experiments. bP<0.05 vs control; eP<0.05 vs H/R.
SFN preserved the cardiomyocytes against H/R injury partly through the attenuation of ER stress-induced apoptosis activation
We then moved to investigate the underlying mechanism of the protective effects offered by SFN. As shown in Figure 4A and 4B, in accordance with ER stress-dependent apoptosis activation, H/R remarkedly suppressed Bcl-2/Bax ratio compared with control group (P<0.05). However, SFN pretrement could elevate the ratio of Bcl-2/Bax (P<0.05).
Sulforaphane (SFN) inhibited ER stress-induced apoptosis and elevated the expression of SIRT1 in cardiomyocytes after H/R injury. (A) The expression of ER stress-related apoptotic proteins were measured by Western blotting. (B–F) ER stress-related apoptosis proteins and SIRT1 were quantified in cardiomyocytes after H/R injury. Data are expressed as Mean±SD of three independent experiments. bP<0.05 vs control; eP<0.05 vs H/R.
As ER stress was often implicated in the activation of apoptosis during H/R injury, we next examined whether SFN preserved cardiomyocytes by modulating ER stress-dependent apoptosis. The levels of ER stress protein markers GRP78, CHOP and cleaved caspase-12 in H/R group were higher than those in H/R group (P<0.05), but lower in SFN and SFN+H/R groups compared with those in H/R group (Figure 4A, 4C, 4D and 4E, P<0.05).
SFN reversed the decrease in mitochondrial membrane potential (ΔΨm) and cell viability of neonatal rat cardiomyocytes after H/R injury
Mitochondria plays an essential function in cell apoptosis during H/R injury. The decline of ΔΨm was regarded as an early event in the apoptotic cascade23. The ΔΨm and cell viability were decreaed after H/R injury. SFN reversed the decrease in ΔΨm and cell viability of neonatal rat cardiomyocytes after H/R injury (P<0.05). Specific SIRT1 inhibitor Ex-527 blocked the effect of SFN (P<0.05, Figure 5).
Effects of sulforaphane (SFN) on mitochondrial membrane potential and cell viability in primary neonatal rat cardiomyocytes undergoing H/R and SIRT1 specific inhibitor Ex-527 (E) blocked the effects of SFN. (A, B) Mitochondrial membrane potential was detected by JC-1 staining in cardiomyocytes. (C) Cardiomyocyte viability was assessed with MTS assay. Data are expressed as Mean±SD of five independent experiments. bP<0.05 vs control; eP<0.05 vs H/R; hP<0.05 vs H/R+SFN.
SFN pretreatment increased the expression of SIRT1 protein
To investigate whether SFN could activate SIRT1 signal pathway, we detected the protein expression of SIRT1 by Western blotting. The expression of SIRT1 increased significantly in SFN pretreated group compared with the H/R group (P<0.05, Figure 4A, 4F). Specific SIRT1 inhibitor Ex-527 could block the effect (P<0.05, Figure 6A and 6F). However, no significant difference in SIRT1 expression was detectable between control group and H/R group (P>0.05, Figure 6A and F). Meanwhile, SFN significantly elevated the ratio of Bcl-2/Bax in cardiomyocytes exposed to H/R injury (P<0.05). Compared with the H/R+SFN group, the expression of SIRT1 and the ratio of Bcl-2/Bax were markedly decreased in H/R+SFN+Ex-527 group (P<0.05, Figure 6A and 6F). Thus, SIRT1 pathway was involved in the anti-apoptotic effects of SFN on neonatal rat cardiomyocytes.
SIRT1 specific inhibitor Ex-527 abolished the protective effects of SFN against ER stress-induced apoptosis in cardiomyocytes after H/R injury. (A) The expression of ER stress-related apoptotic proteins under different conditions. (B–F) ER stress-related apoptosis proteins and SIRT1 were quantified in cardiomyocytes after H/R injury. Data are expressed as Mean±SD of five independent experiments. bP<0.05 vs control; eP<0.05 vs H/R; hP<0.05 vs H/R+SFN.
SIRT1 pathway was involved in modulating ER stress-induced apoptosis activation
To explore whether SIRT1 was involved in the cardioprotective effect of SFN against ER stress, we used SIRT1-specific inhibitor Ex-527 to block SIRT1 pathway. SFN pretreatment decreased the up-regulation of GRP78, CHOP and cleaved caspase-12 expression levels in neonatal rat cardiomyocytes after H/R injury (P<0.05). Ex-527 partially abolished the cardioprotective efffects of SFN against ER stress-induced apoptosis after H/R injury (P<0.05, Figure 6A, 6C-6E).
SFN pretreatment increased SIRT1 activity to attenuate the H/R injury
To further verify our results, we detected the SIRT1 activity in different groups. We found that H/R inhibited the SIRT1 activity compared with control group (P<0.05). SFN pretreatment elevated the downregulation of SIRT1 activity of neonatal rat cardiomyocytes exposed to H/R injury (P<0.05). Ex-527 partially blocked the effect of SFN (P<0.05, Figure 7).
SIRT1 activity under different conditions. (A) SFN could activate SIRT1 activity to attenuate H/R injury; (B) Ex-527 (E) decline SIRT1 activity to abolish the cardioprotective effect of SFN. Data are expressed as Mean±SD of five independent experiments. bP<0.05 vs control; eP<0.05 vs H/R; hP<0.05 vs H/R+SFN.
Discussion
In the present study, we demonstrated SFN could alleviate H/R injury by surpressing ER stress-dependent apoptosis in primary neonatal rat cardiomyocyte. In addition, we further demonstrated that the cardioprotective effects of SFN depended on the activation of SIRT1 signaling pathway, which was shown to suppress ER stress and cardiomyocytes apoptosis in cardiomyocytes exposed to H/R.
(SFN), an isothiocyanate that is present in cruciferous vegetables, has been shown to exert beneficial preventive effect for cardiovascular diseases9,24,25. However, the mechanism still remains unclear. Mounting evidence indicates that apoptosis results in post-ischemic cardiomyocyte death26. In a previous study, SFN could protect cardiomyocytes against oxidative damage27. Our present study found SFN had anti-apoptotic effects in primary neonatal rat cardiomyocytes undergoing H/R. SFN improved cell viability and reduced LDH release in cardiomyocytes subjected to H/R. Anti-apoptotic effect with TUNEL staining assay indicated that SFN might exert a protective effect against apoptosis undergoing H/R. Caspase-3 is one of the key executioners of mammalian cell apoptosis. In this study, SFN also significantly suppressed caspase-3 activity. In short, these data indicated that SFN may have a beneficial effect on cardiomyocytes subjected to H/R.
We investigated the effect of SFN on ER stess in cardiomyocytes undergoing H/R injury. The ER is an important membranous organelles in all eukaryotic cells, which is extremely sensitive to the stimiulation of ischemia and hypoxia28. Studies have shown that ER stress is a pivotal pathophysiological mechanism of myocardial ischemia reperfusion (I/R) injury29,30,31. In addition, some studies demonstrated that SFN reduces ER stress in different models of cell injury. SFN reduces ER stress in type 1 diabetic mouse model accompanying with a reduction of testicular apoptotic cell death32. Similar effects were also observed in human hepatocytes33. Consistent with previous findings, we found that the expression levels of ER stress-associated apoptosis proteins, including GRP78, CHOP and cleaved caspase-12, were upregulated in cardiomyocytes exposed to H/R. Interestingly, pretreatment with SFN decreased the expression levels of GRP78, CHOP and cleaved caspase-12. Meanwhile, the results were in agreement with changes of Bcl-2/Bax ratio. Therefore, the results indicated that SFN partially protected cardiomyocytes against H/R injury via mitigating ER stress-dependent apoptosis.
The anti-apoptotic effects of the SIRT1 have been demonstrated by several studies in myocardial I/R injury. Previous studies have verified that some drugs such as curcumin, melatonin and sildenafil can protect cardiomyocyte against I/R injury by modulating SIRT1 signaling13,34,35. In the present study, we paid attention to the effect of SIRT1 pathways in SFN-elicited protection. First of all, we detected the protein expression of SIRT1 by Western blotting. The expression of SIRT1 increased significantly in SFN pretreated group compared with the H/R group. Specific SIRT1 inhibitor Ex-527 could block the effect. However, there was no significant difference in SIRT1 expression between control group and H/R group. To further verify our results, we detected the SIRT1 activity and found that SFN elevated SIRT1 activity and Ex-527 decreased SIRT1 activity to abrogate the effect of SFN. Moreover, H/R decreased the SIRT1 activity compared with control group. Consistent with our findings, Liu L et al. demonstrated that exogenous NAD+ supplementation restored SIRT1 activity without changing SIRT1 expression under the H/R condition in H9c2 cardiac myoblasts21. Some other studies reported that myocardial I/R insult decreased SIRT1 expression36. It may be resulted from different experimental conditions and model making time37. Our results showed that SFN pretreatment remarkedly elevated the SIRT1 activity compared with the H/R group. Ex-527, the inhibitor of SIRT1, blocked the cardioprotective effect of SFN against apoptotic cell death during H/R injury, including reducing cell viability and increasing apoptotic caridomyocytes by measuring mitochondrial membrane potential. Consistent with previous studies, our study also showed that SIRT1 activation played an essential function in modulating antiapoptic signals by upregulating the ratio of Bcl-2/Bax. In this study, our results indicated that SIRT1 activation and ER stess were closely related during H/R injury-elicited apoptotic process. SFN pretreatment elevated the expression of SIRT1,which was associated with the ER stress. The function of the SIRT1 pathway in the mediation of cardioprotective effect of SFN against H/R-elicited ER stress was further verified by the SIRT1 inhibitor Ex-527. Inhibition of SIRT1 activity contributed to upregulation of ER stress protein markers GRP78, CHOP and Cleaved caspase-12. Moreover, the cardioprotective effect of SFN was suppressed. Therefore, these results manifested that SFN can mitigate ER stress-induced cardiomyocyte apoptosis by activating of SIRT1 signal pathway during H/R injury. However, we should acknowledge that there are still some limitations in the present study. First, our experiment was limited in vitro, which requires further study in vivo. In addition, the underlying mechanisms by which SFN mitigated ER stess remains poorly defined and needs further study.
In conclusion, the study demonstrated that SFN can protect cardiomyocytes against H/R injury and the underlying mechanisms of SFN-mediated cardioprotecion may be dependent on the activation of SIRT1 signaling pathway, which alleviated ER stress-induced apoptosis. Our findings provides a preclinical evidence of the cardioprotective effect of SFN in H/R injury. Due to its efficacy, we suggest that SFN could be considered as a potential pharmacological approach to limit myocardial ischemic reperfusion damage.
Author contribution
Ai-hua CHEN and Li-zi WANG designed the research; Yun-peng LI, Shu-lin WANG, Bei LIU and Lu TANG performed the research; Xian-bao WANG, Cong ZHAO and Xue-ming CAO contributed new analytical tools and reagents; Yun-peng LI, Xu-dong SONG, Xiang WU and Ping-zhen YANG analyzed data; Yun-peng LI wrote the paper.
References
Zhang Y, Ren J . Targeting autophagy for the therapeutic application of histone deacetylase inhibitors in ischemia/reperfusion heart injury. Circulation 2014; 129: 1088–91.
Yao T, Ying X, Zhao Y, Yuan A, He Q, Tong H, et al. Vitamin D receptor activation protects against myocardial reperfusion injury through inhibition of apoptosis and modulation of autophagy. Antioxid Redox Signal 2015; 22: 633–50.
Danilov CA, Chandrasekaran K, Racz J, Soane L, Zielke C, Fiskum G . Sulforaphane protects astrocytes against oxidative stress and delayed death caused by oxygen and glucose deprivation. Glia 2009; 57: 645–56.
Nguyen B, Luong L, Naase H, Vives M, Jakaj G, Finch J, et al. Sulforaphane pretreatment prevents systemic inflammation and renal injury in response to cardiopulmonary bypass. J Thorac Cardiovasc Surg 2014; 148: 690–7.
Forster T, Rausch V, Zhang Y, Isayev O, Heilmann K, Schoensiegel F, et al. Sulforaphane counteracts aggressiveness of pancreatic cancer driven by dysregulated Cx43-mediated gap junctional intercellular communication. Oncotarget 2014; 5: 1621–34.
Ho JN, Yoon HG, Park CS, Kim S, Jun W, Choue R, et al. Isothiocyanates ameliorate the symptom of heart dysfunction and mortality in a murine AIDS model by inhibiting apoptosis in the left ventricle. J Med Food 2012; 15: 781–7.
Zhu H, Jia Z, Strobl JS, Ehrich M, Misra HP, Li Y . Potent induction of total cellular and mitochondrial antioxidants and phase 2 enzymes by cruciferous sulforaphane in rat aortic smooth muscle cells: cytoprotection against oxidative and electrophilic stress. Cardiovasc Toxicol 2008; 8: 115–25.
Angeloni C, Leoncini E, Malaguti M, Angelini S, Hrelia P, Hrelia S . Modulation of phase II enzymes by sulforaphane: implications for its cardioprotective potential. J Agric Food Chem 2009; 57: 5615–22.
Piao CS, Gao S, Lee GH, Kim DS, Park BH, Chae SW, et al. Sulforaphane protects ischemic injury of hearts through antioxidant pathway and mitochondrial KATP channels. Pharmacol Res 2010; 61: 342–8.
Yeh CH, Chen TP, Wang YC, Lin YM, Fang SW . AMP-activated protein kinase activation during cardioplegia-induced hypoxia/reoxygenation injury attenuates cardiomyocytic apoptosis via reduction of endoplasmic reticulum stress. Mediators Inflamm 2010; 2010: 130636.
Pan C, Prentice H, Price AL, Wu JY . Beneficial effect of taurine on hypoxia- and glutamate-induced endoplasmic reticulum stress pathways in primary neuronal culture. Amino Acids 2012; 43: 845–55.
Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R . The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther 2008; 120: 172–85.
Yang Y, Duan W, Lin Y, Yi W, Liang Z, Yan J, et al. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic Biol Med 2013; 65: 667–79.
Guo Z, Liao Z, Huang L, Liu D, Yin D, He M . Kaempferol protects cardiomyocytes against anoxia/reoxygenation injury via mitochondrial pathway mediated by SIRT1. Eur J Pharmacol 2015; 761: 245–53.
Carloni S, Albertini MC, Galluzzi L, Buonocore G, Proietti F, Balduini W . Melatonin reduces endoplasmic reticulum stress and preserves sirtuin 1 expression in neuronal cells of newborn rats after hypoxia-ischemia. J Pineal Res 2014; 57: 192–9.
Lu FH, Fu SB, Leng X, Zhang X, Dong S, Zhao YJ, et al. Role of the calcium-sensing receptor in cardiomyocyte apoptosis via the sarcoplasmic reticulum and mitochondrial death pathway in cardiac hypertrophy and heart failure. Cell Physiol Biochem 2013; 31: 728–43.
Zhang Q, Huang WD, Lv XY, Yang YM . Ghrelin protects H9c2 cells from hydrogen peroxide-induced apoptosis through NF-kappaB and mitochondria-mediated signaling. Eur J Pharmacol 2011; 654: 142–9.
Wang M, Meng XB, Yu YL, Sun GB, Xu XD, Zhang XP, et al. Elatoside C protects against hypoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes through the reduction of endoplasmic reticulum stress partially depending on STAT3 activation. Apoptosis 2014; 19: 1727–35.
Jia Y, Zuo D, Li Z, Liu H, Dai Z, Cai J, et al. Astragaloside IV inhibits doxorubicin-induced cardiomyocyte apoptosis mediated by mitochondrial apoptotic pathway via activating the PI3K/Akt pathway. Chem Pharm Bull 2014; 62: 45–53.
Bassino E, Fornero S, Gallo MP, Gallina C, Femmino S, Levi R, et al. Catestatin exerts direct protective effects on rat cardiomyocytes undergoing ischemia/reperfusion by stimulating PI3K-AKT-GSK3beta pathway and preserving mitochondrial membrane potential. PLoS One 2015; 10: e119790.
Liu L, Wang P, Liu X, He D, Liang C, Yu Y . Exogenous NAD+ supplementation protects H9c2 cardiac myoblasts against hypoxia/reoxygenation injury via Sirt1-p53 pathway. Fundam Clin Pharmacol 2014; 28: 180–9.
Duan JL, Wang JW, Guan Y, Yin Y, Wei G, Cui J, et al. Safflor yellow A protects neonatal rat cardiomyocytes against anoxia/reoxygenation injury in vitro. Acta Pharmacol Sin 2013; 34: 487–95.
Sun X, Sun GB, Wang M, Xiao J, Sun XB . Protective effects of cynaroside against H2O2-induced apoptosis in H9c2 cardiomyoblasts. J Cell Biochem 2011; 112: 2019–29.
Li Z, Galli U, Becker LE, Bruns H, Nickkolgh A, Hoffmann K, et al. Sulforaphane protects hearts from early injury after experimental transplantation. Ann Transplant 2013; 18: 558–66.
Wu QQ, Zong J, Gao L, Dai J, Yang Z, Xu M, et al. Sulforaphane protects H9c2 cardiomyocytes from angiotensin II-induced hypertrophy. HERZ 2014; 39: 390–6.
Wang C, Chen K, Xia Y, Dai W, Wang F, Shen M, et al. N-Acetylcysteine attenuates ischemia-reperfusion-induced apoptosis and autophagy in mouse liver via regulation of the ROS/JNK/Bcl-2 pathway. PLoS One 2014; 9: e108855.
Leoncini E, Malaguti M, Angeloni C, Motori E, Fabbri D, Hrelia S . Cruciferous vegetable phytochemical sulforaphane affects phase II enzyme expression and activity in rat cardiomyocytes through modulation of Akt signaling pathway. J Food Sci 2011; 76: H175–81.
Xie P, Duan Y, Guo X, Hu L, Yu M . SalA attenuates hypoxia-induced endothelial endoplasmic reticulum stress and apoptosis via down-regulation of VLDL receptor expression. Cell Physiol Biochem 2015; 35: 17–28.
Sano R, Reed JC . ER stress-induced cell death mechanisms. Biochim Biophys Acta 2013; 1833: 3460–70.
Sheng R, Liu XQ, Zhang LS, Gao B, Han R, Wu YQ, et al. Autophagy regulates endoplasmic reticulum stress in ischemic preconditioning. Autophagy 2012; 8: 310–25.
Zheng D, Wang G, Li S, Fan GC, Peng T . Calpain-1 induces endoplasmic reticulum stress in promoting cardiomyocyte apoptosis following hypoxia/reoxygenation. Biochim Biophys Acta 2015; 1852: 882–92.
Jiang X, Bai Y, Zhang Z, Xin Y, Cai L . Protection by sulforaphane from type 1 diabetes-induced testicular apoptosis is associated with the up-regulation of Nrf2 expression and function. Toxicol Appl Pharmacol 2014; 279: 198–210.
He C, Li B, Song W, Ding Z, Wang S, Shan Y . Sulforaphane attenuates homocysteine-induced endoplasmic reticulum stress through Nrf-2-driven enzymes in immortalized human hepatocytes. J Agric Food Chem 2014; 62: 7477–85.
Yu L, Sun Y, Cheng L, Jin Z, Yang Y, Zhai M, et al. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J Pineal Res 2014; 57: 228–38.
Shalwala M, Zhu SG, Das A, Salloum FN, Xi L, Kukreja RC . Sirtuin 1 (SIRT1) activation mediates sildenafil induced delayed cardioprotection against ischemia-reperfusion injury in mice. PLoS One 2014; 9: e86977.
Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 2014; 122: 2170–82.
Hu ZY, Peng XY, Liu F, Liu J . Emulsified isoflurane protects rat heart in situ after regional ischemia and reperfusion. Fundam Clin Pharmacol 2014; 28: 190–8.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No 81270218 to Dr Ai-hua CHEN and No 81400190 to Dr Xian-bo WANG) and Science and Technology Project of Guangdong Province (No 2014A020212191 to Dr Ping-zhen YANG).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, Yp., Wang, Sl., Liu, B. et al. Sulforaphane prevents rat cardiomyocytes from hypoxia/reoxygenation injury in vitro via activating SIRT1 and subsequently inhibiting ER stress. Acta Pharmacol Sin 37, 344–353 (2016). https://doi.org/10.1038/aps.2015.130
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2015.130
Keywords
This article is cited by
-
Dapagliflozin attenuates pressure overload-induced myocardial remodeling in mice via activating SIRT1 and inhibiting endoplasmic reticulum stress
Acta Pharmacologica Sinica (2022)
-
Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases
Nature Reviews Cardiology (2021)
-
Unfolded protein response during cardiovascular disorders: a tilt towards pro-survival and cellular homeostasis
Molecular and Cellular Biochemistry (2021)
-
Dexmedetomidine inhibits endoplasmic reticulum stress to suppress pyroptosis of hypoxia/reoxygenation-induced intestinal epithelial cells via activating the SIRT1 expression
Journal of Bioenergetics and Biomembranes (2021)