Abstract
Aim:
To investigate the anti-tumor effects of α-mangostin, a major xanthone identified in the pericarp of mangosteen (Garcinia mangostana Linn), against human gastric adenocarcinoma cells in vitro, and the mechanisms of the effects.
Methods:
Human gastric adenocarcinoma cell lines BGC-823 and SGC-7901 were treated with α-mangostin. The cell viability was measured with MTT assay, and cell apoptosis was examined using flow cytometry and TUNEL assay. The expression of the relevant proteins was detected using Western blot.
Results:
Treatment with α-mangostin (3–10 μg/mL) inhibited the viability of both BGC-823 and SGC-7901 cells in dose- and time-manners. Furthermore, α-mangostin (7 μg/mL) time-dependently increased the apoptosis index of the cancer cells, reduced the mitochondrial membrane potential of the cancer cells, and significantly increased the release of cytochrome c and AIF into cytoplasm. Moreover, the α-mangostin treatment markedly suppressed the constitutive Stat3 protein activation, and Stat3-regulated Bcl-xL and Mcl-1 protein levels in the cancer cells.
Conclusion:
The anti-tumor effects of α-mangostin against human gastric adenocarcinoma cells in vitro can be partly attributed to blockade of Stat3 signaling pathway.
Similar content being viewed by others
Introduction
Gastric adenocarcinoma remains among the most aggressive and lethal diseases worldwide, with gastric adenocarcinoma being the second leading cause of cancer-related deaths1,2. Currently, 5-FU, a first-line treatment for advanced gastric adenocarcinoma, is extensively utilized but offers only limited benefits because of acquired chemoresistance and multiple adverse effects3,4,5. Thus, the development of effective therapeutic approaches for gastric adenocarcinoma remains one of the most challenging goals in cancer research. The potential of some natural products in cancer therapy make such products promising candidates for use in chemoprevention regimens and as novel adjunctive agents to fill a critical need in the effective, safe, and less invasive treatment of cancer.
Many tropical plants have interesting biological activities with potential therapeutic applications6. Based on traditional usage and subsequent scientific findings, mangosteen (Garcinia mangostana Linn) is thought to be a potential anti-cancer agent. Mangosteen has a wide variety of pharmacologic activities. The major therapeutic benefits of mangosteen have been attributed to xanthone-type compounds7,8,9. Indeed, as of 2012, more than 68 xanthone-type compounds derived from mangosteen have been reported. The biological activities of α-mangostin have been confirmed to consist of anti-proliferative and apoptotic effects on various cancer cells, such as human leukemia, hepatomas, lung, human breast, and colorectal cancers10,11,12,13,14. The molecular mechanisms for these α-mangostin biological effects are not fully defined, although several reports indicate that the apoptotic effect of α-mangostin is the principal pathway of mediation.
The signal transducer and activator of transcription (STAT) family of cytoplasmic transcription factors has been shown to participate in numerous processes that are key to malignant progression, including proliferation and metastasis15,16,17. STAT is activated by the phosphorylation of a conserved tyrosine residue in response to extracellular signals and oncogenes and involves dimerization between two phosphorylated STAT monomers, followed by the translocation of the dimers into the nucleus. STAT dimers bind to specific DNA response elements in the promoters of target genes in the nucleus and regulate their expression18,19. Although normal STAT activation is highly regulated and transient, one member of the STAT family, Stat3, is constitutively activated in diverse human tumors, including gastric adenocarcinoma, largely because of hyperactive tyrosine kinases20,21,22,23,24. Constitutively active Stat3 induces oncogenic processes, such as dysregulated growth, survival, angiogenesis, and immune modulation, thereby contributing to malignant transformation and progression.
Although the anti-proliferative role of α-mangostin in malignant diseases has been increasingly recognized, the precise cellular mechanism by which α-mangostin serves a function in cancer remains unknown. Given the collective roles of Stat3 in many human tumors, whether or not the potential anti-cancer role of α-mangostin is associated with Stat3 signaling remains unclear. We discovered that α-mangostin inhibits Stat3 activation in gastric adenocarcinoma cells and represses cell proliferation along with apoptosis. Thus, our studies provide a novel potential application of α-mangostin as a small-molecule inhibitor of Stat3 signaling with antitumor cell activity.
Materials and methods
Cell cultures and treatments
The human gastric adenocarcinoma cell lines BGC-823 and SGC-7901 (obtained from the American Tissue Type Collection, USA) were maintained in Dulbecco's modified Eagle's medium (DMEM, GIBCO, USA) supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), 0.1 mmol/L nonessential amino acids, 0.2 mmol/L glutamine, 1 mmol/L pyruvate, and 10% heat-inactivated fetal bovine serum (FBS) and then incubated in 5% CO2 humidified atmosphere at 37 °C. Cells were grown to 80% confluency prior to treatment. The antibodies against pSTAT3, STAT3, Bcl-xL, Mcl-1, cytochrome c, AIF, and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Annexin V-FITC and propidium iodide (PI), α-mangostin (Xanomax 95™, 95%) was obtained from Avesthagen (Chatsworth, CA, USA).
Proliferation assay
Cell proliferation was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT Sigma) uptake method. The cells were seeded (5×103/well) in 200 μL of DMEM medium into 96-well plates and cultured overnight. After treatment with α-mangostin (0 μg/mL to 10 μg/mL) at 37 °C with 5% CO2, MTT reagent (5 mg/mL) was added at the time of cell growth evaluation, and incubation was continued for an additional 4 h. The reaction was terminated with 150 μL of dimethylsulfoxide (DMSO, Sigma, USA) per well. Absorbance values were determined using an MRX Revelation 96-well multiscanner (Dynex Technologies, Chantilly, VA, USA). The cells cultured in DMEM served as the control. The cell viability index was calculated according to the following formula: experimental OD value/control OD value. The experiments were repeated thrice.
Detection of cellular apoptosis by flow cytometry
Apoptosis was evaluated with an Annexin V–FITC/PI apoptosis detection kit according to manufacturer's instructions. Cells were seeded (105/well) in 6-well plates in DMEM for 24 h. The medium was removed, cells were washed with PBS, and then α-mangostin (7 μg/mL) was added. At different time points, cells were trypsinized and centrifuged, washed with PBS, and stained with Annexin V and propidium iodide in the dark. Samples were analyzed, and the percentage of apoptotic cells was evaluated using the FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA, USA).
In situ detection of apoptotic cells
TUNEL assays were performed with an in situ cell apoptosis detection kit following manufacturer's instructions. Briefly, the cells were placed on cover slides after exposure to α-mangostin at different time points and then fixed with 4% paraformaldehyde for 30 min. The nonspecific chromogen reaction, induced by endogenous peroxidase, was inhibited with 3% H2O2 for 10 min. Terminal deoxynucleotidyl transferases (TdT) were used for the incorporation of DNA strand breaks in situ for 1 h at 37 °C in a humidified box. Positive control slides were treated with DNase, whereas negative control slides were treated with PBS instead of TdT. DNA fragments were stained using DAB as a substrate for peroxidase, and hematoxylin was used as a counter stain. The apoptotic index was calculated as a ratio of the number of apoptotic cells to the total number of tumor cells in each slide.
Analysis of mitochondrial membrane potential
The analysis was conducted using JC-1 according to manufacturer's instructions. Gastric adenocarcinoma cells were seeded in 6-well plates at a density of 4×105 cells per well and were treated with or without α-mangostin (7 μg/mL) for the indicated periods. Cells were washed with PBS and stained with 2 μg/mL of JC-1 for 20 min at 37 °C. Cells were washed with PBS twice, resuspended with PBS, and analyzed by the FACSCalibur flow cytometer.
Transmission electron microscopy
To evaluate the morphological features of cell death, the cells in the control group or cells treated with or without α-mangostin were processed for transmission electron microscopy (TEM). Briefly, cells were fixed for 30 min in 4% paraformaldehyde and 1% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.4) (PB), rinsed in PB, and post-fixed in 1% osmium tetraoxide for 30 min. After washing in PB, cells were progressively dehydrated in a 10% graded series of 50% to 100% ethanol and then cleared in QY-1 (Nissin EM, Tokyo Japan). Cells were embedded in Epon 812 resin. Thin sections (70 nm thickness) were stained with uranyl acetate and lead citrate and then examined by TEM.
Immunofluorescence assay
Exponentially growing cells were seeded on 25-mm square glass cover slips and placed in 35 mm diameter culture dishes. After treatment, the cells were fixed with 4% formaldehyde for 5 min, permeabilized with 0.2% solution of Triton X-100 in PBS, and blocked with 2% bovine serum albumin-PBS for 30 min. Slides were incubated with anti-STAT3 and anti-pSTAT3 overnight. Fluorescent imaging was performed with a confocal laser scanning microscope (Carl Zeiss MicroImaging, Inc, USA).
Western blot analysis
Briefly, 5×105 cells were incubated for 30 min in 0.5 mL of ice-cold whole-cell lysate buffer (10% NP40, 5 mol/L NaCl, 1 mol/L HEPES, 0.1 mol/L EGTA, 0.5 mol/L EDTA, 0.1 mol/L PMSF, 0.2 mol/L sodium orthovanadate, 1 mol/L NaF, 2 μg/mL aprotinin, and 2 μg/mL leupeptin). Debris was removed by centrifugation. The protein content of the cell was determined, and the cellular lysates were separated by 10% SDS-PAGE and then electro-transferred onto nitrocellulose membranes. After being blocked with 5% non-fat milk in TBST (20 mmol/L Tris, 150 mmol/L NaCl, 0.2% Tween-20, pH 7.6), the membranes were incubated with primary antibodies at 4 °C overnight, followed by 1:2000 horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz) for 2 h. Immunoreactive bands were visualized using an enhanced chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Statistical analysis
Each experiment was performed at least thrice. Data were expressed in mean values±standard deviation. Data were analyzed by chi-square test (χ2), two-sided Fisher exact test, or one-way ANOVA tests. P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS Version 13.0 statistical software (SPSS, Chicago, Illinois, USA).
Results
Effect of α-mangostin on the growth of gastric adenocarcinoma cells
We initially investigated the effect of α-mangostin on the proliferation of BGC-823 and SGC-7901 cells using the MTT assay. Both gastric adenocarcinoma cell lines were treated with graded concentrations of α-mangostin (0 to 10 μg/mL) for 6, 12, 18, 24, and 48 h. As shown in Figure 1A, cell proliferation was inhibited by α-mangostin treatment in a time- and concentration-dependent manner. Compared with 0 μg/mL (DMSO treatment alone), cell proliferation was not significantly altered at a concentration of α-mangostin (≤3 μg/mL), indicating that α-mangostin is not toxic to BGC-823 cells at these dosages. When cells were treated with 5 to 10 μg/mL, cell viability was significantly decreased. The same result was observed in SGC-7901 cells, although these cells were less sensitive to the cytotoxic effect of α-mangostin than the BGC-823 cells (Figure 1B). Thus, α-mangostin induces potent growth inhibition in gastric adenocarcinoma cells. The optimal concentration 7 μg/mL of α-mangostin was therefore used for subsequent experiments.
Anti-proliferative effect of α-mangostin (0 to 10 μg/mL) at different time points. (A) Anti-proliferative effect of α-mangostin in BGC-823 gastric adenocarcinoma cells; (B) Anti-proliferative effect of α-mangostin in SGC-7901 gastric adenocarcinoma cells. The results are representative of three independent experiments. bP<0.05 vs untreated group.
Apoptosis induced by α-mangostin in gastric adenocarcinoma cells
To establish whether the anti-proliferation effect of α-mangostin is induced by apoptosis, we tested the apoptotic rate of two cell lines with a flow cytometry assay. BGC-823 and SGC-7901 cells were treated with α-mangostin (7 μg/mL) for 6, 18, and 24 h. Alpha-mangostin increased the apoptosis index in a time-dependent manner compared with control (P<0.05; Figure 2A).
(A) Effect of α-mangostin on cellular apoptosis. BGC-823 and SGC-7901 cells were incubated with α-mangostin (7 μg/mL). After 6, 18, and 24 h, cellular apoptosis was examined for by Annexin V-PI double-labeling and FACS analysis. (B) TUNEL assay showing the apoptotic effect of α-mangostin on cells. BGC-823 and SGC-7901 cells were incubated with α-mangostin (7 μg/mL) for 6, 18, and 24 h. The results are representative of three independent experiments. (C) Alpha-mangostin-induced typical chromatin offset and apoptotic body formation gradually in BGC-823 and SGC-7901 cells. Cells were incubated with α-mangostin (7 μg/mL) after 6, 18, and 24 h, and cellular morphologic changes were examined by transmission electron microscopy (×6000–10000). The results are representative of three independent experiments.
To confirm this phenomenon further, we sequentially conducted the TUNEL assay. These results were in agreement with the flow cytometry assay and revealed that α-mangostin significantly increased the rate of apoptosis in a time-dependent manner (Figure 2B). Moreover, TEM observations indicated that both cell types gradually underwent typical chromatin offset and apoptotic body formation (Figure 2C). Thus, α-mangostin has inhibitory effects on cellular proliferation associated with apoptosis.
Mitochondrial dysfunctions in α-mangostin-treated cells
Mitochondrial function reportedly precedes apoptosis protein activation25,26. To understand whether α-mangostin induces gastric adenocarcinoma cell apoptosis via a mitochondrial pathway, we investigated various parameters of mitochondrial dysfunction 3 h after treatment with α-mangostin (7 μg/mL). Electron microscopic observations indicated mitochondrial swelling 3 h after incubation with α-mangostin (Figure 3A, 3B). The release of cytochrome c (Cyt.c) and AIF into the cytoplasm was detected after 3 h (Figure 3C, 3D, 3G, 3H; P<0.05). Decreases in ΔΨm (Figures 3E, 3F) were also observed 3 h after treatment with α-mangostin using a flow cytometry assay (P<0.05). These results indicated that mitochondrial dysfunction is induced by α-mangostin and is involved in gastric adenocarcinoma cell apoptosis.
Mitochondrial dysfunction in α-mangostin-treated cells. Electron microscopic observations indicated mitochondrial swelling 3 h after incubation with α-mangostin in BGC-823 (A) and SGC-7901 (B) cells. The release of cytochrome c (Cyt.c) and AIF into cytoplasm was detected at 3 h by Western blotting in BGC-823 (C) and SGC-7901 (D) cells, respectively. (G, H) quantification of Western blotting data. Decreases in ΔΨm were also observed 3 h after the treatment with α-mangostin by flow cytometry in BGC-823 (E) and SGC-7901 (F) cells. Data from at least three independent experiments with duplicate determinations are expressed as the mean±SEM. bP<0.05 vs control.
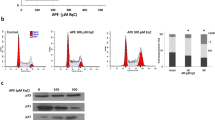
Alpha-mangostin inhibits Stat3 activation in gastric adenocarcinoma cells
To understand the molecular mechanisms underlying α-mangostin-induced gastric adenocarcinoma cell apoptosis, an immunofluorescence assay was performed to determine STAT3 and pSTAT3 alterations in SGC-7901 and BGC-823 cells treated with α-mangostin and stained with TRITC. The cells were also analysed by confocal microscopy. After 24 h, the pSTAT3 fluorescence signal in the α-mangostin group was lower than that in the control group, whereas no difference was observed between two groups in terms of STAT3 expression (Figures 4A, 4B). To confirm pSTAT3 alteration further, we performed a sequential test using Western blot analysis. The results were in agreement with those of the immunofluorescence assay (Figures 4C–4F). These findings further indicate that the STAT3 signaling pathway is involved in the anti-tumor effects of α-mangostin.
(A, B) Immunodetection of Stat3 and pStat3 protein in gastric adenocarcinoma cells. BGC-823 and SGC-7901 cells were incubated with α-mangostin (7 μg/mL). After 24 h, fluorescent imaging was obtained with a confocal laser scanning microscope. The pSTAT3 fluorescence signal in α-mangostin group was lower than the control group, whereas the STAT3 fluorescence signal was not different between groups. (C, D) Western blot analyses of STAT3, p-STAT3. (E, F) Quantification of Western blot data. Data are representative of three independent assays. bP<0.05 vs control.
Alpha-mangostin blocks Bcl-xL and Mcl-1 expression in gastric adenocarcinoma cells
To elucidate further the mechanism by which α-mangostin-mediated inhibition of constitutive Stat3 activation results in cell growth inhibition and apoptosis, we examined the anti-apoptotic proteins, Bcl-xL and Mcl-1, both of which are target genes for Stat3. Using SGC-7901 and BGC-823 cells treated with α-mangostin, Western blot analysis showed significant reductions in the levels of expression of Bcl-xL and Mcl-1 (Figure 5), which correlates with the inhibition of Stat3 phosphorylation and activity by α-mangostin (Figure 4). These data suggest that α-mangostin induces cell growth inhibition and apoptosis of tumor cells, at least in part, by inhibiting Stat3-depedent induction of Bcl-xL and Mcl-1.
Alpha-mangostin blocks Bcl-xL and Mcl-1 expression in gastric adenocarcinoma cells. BGC-823 (A) and SGC-7901 (B) cells were treated with or without α-mangostin (7 μg/mL) for 6, 18, and 24 h, from which lysates were prepared. Western blot analyses of cell lysates probed for Bcl-xL and Mcl-1. (C, D) Quantification of Western blotting data. Data are representative of three independent assays. bP<0.05 vs control.
Discussion
A number of studies describing tumor preventive and therapeutic activities of dietary phytochemicals have been recently reported27. Although the putative cellular targets and the mechanisms of some compounds extracted from plants, including α-mangostin, have been identified, the precise molecular mechanisms remain unclear. In this study, we investigated the effects of α-mangostin on cell proliferation in gastric adenocarcinoma cell lines. We found that α-mangostin inhibits the proliferation of gastric adenocarcinoma cells in a time- and concentration-dependent manner by inactivating the STAT3 signaling pathway. Blocking STAT3 inhibited Bcl-xL and Mcl-1 expression and enhanced apoptotic effects. Collectively, our in vitro evidence extend our understanding of the function of α-mangostin and show that the anti-cancer properties of α-mangostin can be partly attributed to the blockade of constitutive Stat3 activities, thus suggesting that STAT3 may be a potential strategy for α-mangostin therapy.
Cancer remains one of the most aggressive and lethal diseases worldwide. Although surgery, chemotherapy, and radiotherapy have been standard cancer treatment options for many years, these anti-cancer therapies can only offer modest benefits for cancer patients with metastases, acquired chemoresistance, and toxicity28,29,30. Consequently, cancer prevention using non-toxic chemical entities, commonly termed “chemoprevention,” is a more realistic and fundamental strategy for the management of this disease. Manogosteen, a well-known tropical fruit, exhibits a wide variety of pharmacologic activities. The pericarp of the fruit contains considerable amounts of biologically active compounds, such as xanthones, terpenes, anthocyanins, tannins, and phenols9. Alpha-mangostin, β-mangostin, γ-mangostin, garcinone E, and gartanin, which are xanthone-type compounds, have potential chemopreventive and chemotherapeutic properties that have been extensively investigated for their inhibitory effect on every step of the carcinogenesis process. These compounds can inhibit several molecular targets in the tumor cells, including kinases, cyclooxygenases, ribonucleotide reductase, and DNA polymerases14,31,32. The anti-tumor activities of xanthones include cell cycle arrest, suppression of tumor cell proliferation, induction of apoptosis and differentiation, reduction of inflammation, and inhibition of adhesion, invasion, and metastasis. The anti-tumor activity of xanthones was first observed in Raji and P3HR-1 lymphoblastoid cells and subsequently noted in HL60, K562, NB4, and U937 leukemia cells33. In leukemia cells, α-, β-, and γ-mangostin are effective, despite the use of a low dose (4.17 μg/mL). Of these compounds, α-mangostin demonstrated the strongest inhibitory activity in all cell lines tested, particularly in HL60, NB4, and U937 cells, with complete suppression of cell growth 72 h following treatment. A significant cytotoxic effect was not observed on peripheral blood lymphocytes. Thus, α-mangostin appears to target leukemia cells preferentially33. Based on the MTT assay, we did not observe a decrease in cell viability of gastric adenocarcinoma cells treated with α-mangostin (<3 μg/mL). In two different studies with a focus on cardioprotection, α-mangostin was administered (200 mg/kg by oral gavage) to rats for up to 8 d with no observable adverse effects in solid organ systems34,35. In another study, α-mangostin was tolerable with no observable adverse effects during a five-week intervention with custom-blended food pellets that contained 0.02 or 0.05% α-mangostin in F344 rats36.
Apoptosis of tumor cells can be triggered by a diversity of extracellular and intracellular factors, including cytokines, tumor suppressor genes, oncogenes, radiation, and anti-cancer drugs. Mitochondria serve an essential function in various forms of apoptosis25. Various apoptotic stimulus signals through Bcl-2 homology in domain 3 (BH3) proteins cause a Bcl-2-associated protein X (Bax) protein shift to the outer mitochondrial membrane, form a membrane channel, and then stimulate the release of cytochrome c from the inner mitochondrial membrane to the cytosol, thus triggering the assembly of the apoptosome and leading to a cascade of downstream caspases. Apoptosis inhibitor protein regulates apoptosis by inhibiting the caspase cascade26. We determined whether mitochondria are involved in α-mangostin-induced apoptosis. Treatment of BGC-823 and SGC-7901 cells with α-mangostin caused rapid mitochondrial membrane depolarization and cytochrome c release. These results suggest that α-mangostin induces apoptosis via the mitochondrial pathway. Another report showed that mitochondria are preferential targets in α-mangostin-induced apoptosis in human leukemia HL60 cells33.
The abovementioned bioactivity mechanism of α-mangostin may involve the suppression of the activity of Stat3. Stat3 regulates a number of important tumorigenesis pathways, including cell cycle progression, apoptosis, tumor angiogenesis, and tumor cell evasion of the immune system20,37,38,39. In this study, we demonstrated that Stat3 can be inactivated by α-mangostin, which can also downregulate the downstream gene expression of Bcl-xL and Mcl-1. Thus, Stat3 signaling can be a potential therapeutic target of α-mangostin in managing tumors. However, further studies are required to examine the clinical relevance of this finding.
In conclusion, our results reveal that α-mangostin inhibits proliferation and potentiates cell apoptosis in gastric adenocarcinoma cells, likely by inhibiting activation of STAT3 and the expression of its regulated genes. Our study provides novel potential applications for α-mangostin as a small-molecule inhibitor of Stat3 signaling with anti-tumor cell activity.
Author contribution
Tao SHAN, Xi-juan CUI, Yi-ming LI, and Xi CHEN designed research; Tao SHAN, Xi-juan CUI, Wei LI, Wan-run LIN, and Hong-wei LU performed research; Yi-ming LI, Xi CHEN and Tao WU contributed part reagents; Tao SHAN and Xi-juan CUI analyzed data; Tao SHAN and Xi-juan CUI wrote the paper.
References
Brunner T . Gemcitabine in the chemoradiotherapy for locally advanced pancreatic cancer: a meta-analysis. Strahlenther Onkol 2012; 188: 366–7.
Kim J, Lim JH, Kim JH, Im S, Chie EK, Hwang J, et al. Phase II clinical trial of induction chemotherapy with fixed dose rate gemcitabine and cisplatin followed by concurrent chemoradiotherapy with capecitabine for locally advanced pancreatic cancer. Cancer Chemoth Pharm 2012; 70: 381–9.
Kido H, Morizane C, Tamura T, Hagihara A, Kondo S, Ueno H, et al. Gemcitabine-induced pleuropericardial effusion in a patient with pancreatic cancer. Jpn J Clin Oncol 2012; 42: 845–50.
Chang Y, Yu C, Chen C, Hu F, Hsu H, Tseng WI, et al. Dynamic contrast-enhanced MRI in advanced nonsmall-cell lung cancer patients treated with first-line bevacizumab, gemcitabine, and cisplatin. J Magn Reson Imaging 2012; 36: 387–96.
Li Y, Gu W, Liu H . Induction of pancreatic cancer cell apoptosis and enhancement of gemcitabine sensitivity by rap80 sirna. Digest Dis Sci 2012; 57: 2072–8.
Surh YJ . Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 2003; 3: 768–80.
Marcason W . What are the facts and myths about mangosteen? J Am Diet Assoc 2006; 106: 986.
Suksamrarn S, Komutiban O, Ratananukul P, Chimnoi N, Lartpornmatulee N, Suksamrarn A . Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem Pharm Bull 2006; 54: 301–5.
Yeung S . Mangosteen for the cancer patient: facts and myths. J Soc Integr Oncol 2006; 4: 130–4.
Matsumoto K, Akao Y, Ohguchi K, Ito T, Tanaka T, Iinuma M, et al. Xanthones induce cell-cycle arrest and apoptosis in human colon cancer DLD–1 cells. Bioorgan Med Chem 2005; 13: 6064–9.
Matsumoto K, Akao Y, Yi H, Ohguchi K, Ito T, Tanaka T, et al. Preferential target is mitochondria in alpha-mangostin-induced apoptosis in human leukemia HL60 cells. Bioorgan Med Chem 2004; 12: 5799–806.
Sato A, Fujiwara H, Oku H, Ishiguro K, Ohizumi Y . alpha–Mangostin induces Ca2+-ATPase-dependent apoptosis via mitochondrial pathway in PC12 cells. J Pharmacol Sci 2004; 94: 138P.
Nabandith V, Suzui M, Morioka T, Kaneshiro T, Kinjo T, Matsumoto K, et al. Inhibitory effects of crude alpha-mangostin, a xanthone derivative, on two different categories of colon preneoplastic lesions induced by 1,2-dimethylhydrazine in the rat. Asian Pac J Cancer Prev 2004; 5: 433–8.
Moongkarndi P, Kosem N, Kaslungka S, Luanratana O, Pongpan N, Neungton N . Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J Ethnopharmacol 2004; 90: 161–6.
Anonymous. Common stem: stat3. Prescrire Int 2013; 22: 11.
Ginter T, Heinzel T, Kramer OH . Acetylation of endogenous STAT proteins. Methods Mol Biol 2013; 967: 167–78.
Sun Y, Cheng M, Griffiths TRL, Mellon JK, Kai B, Kriajevska M, et al. Inhibition of STAT signalling in bladder cancer by diindolylmethane — relevance to cell adhesion, migration and proliferation. Curr Cancer Drug Tar 2013; 13: 57–68.
Kang K, Robinson GW, Hennighausen L . Comprehensive meta-analysis of signal transducers and activators of transcription (STAT) genomic binding patterns discerns cell-specific cis-regulatory modules. BMC Genomics 2013; 14.
Subramaniam A, Shanmugam MK, Perumal E, Li F, Nachiyappan A, Dai X, et al. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Bba-Rev Cancer 2013; 1835: 46–60.
Dobi E, Monnien F, Kim S, Ivanaj A, N'Guyen T, Demarchi M, et al. Impact of STAT3 phosphorylation on the clinical effectiveness of anti-EGFR-based therapy in patients with metastatic colorectal cancer. Clin Colorec Cancer 2013; 12: 28–36.
Mohanty SK, Yagiz K, Cinar B, Amin MB, Luthringer D, Alkan S . STAT3 and STAT5a are potential therapeutic targets in castration-resistant prostate cancer. Lab Invest 2013; 931: 444a.
Guo J, Kim D, Gao J, Kurtyka C, Chen H, Yu C, et al. IKBKE is induced by STAT3 and tobacco carcinogen and determines chemosensitivity in non–small cell lung cancer. Oncogene 2013; 32: 151–9.
Nagathihalli N, Beesetty Y, Shi C, Merchant N . Cross–talk between STAT3 and MAPK signaling in pancreatic cancer. Pancreas 2012; 41: 1389.
Liu A, Liu Y, Jin Z, Hu Q, Lin L, Jou D, et al. XZH–5 inhibits STAT3 phosphorylation and enhances the cytotoxicity of chemotherapeutic drugs in human breast and pancreatic cancer cells. PloS One 2012; 7 (e4662410).
Lemarie A, Grimm S . Mitochondrial respiratory chain complexes: apoptosis sensors mutated in cancer. Oncogene 2011; 30: 3985–4003.
Fulda S . Exploiting mitochondrial apoptosis for the treatment of cancer. Mitochondrion 2010; 10: 598–603.
Erbersdobler H . What role do dietary factors play in cancer prevention? Ernahrungs–Umschau 2008; 55: 606–7.
Amin S, McBride RB, Kline JK, Mitchel EB, Lucas AL, Neugut AI, et al. Incidence of subsequent pancreatic adenocarcinoma in patients with a history of nonpancreatic primary cancers. Cancer 2012; 118: 1244–51.
Lambe M, Eloranta S, Wigertz A, Blomqvist P . Pancreatic cancer; reporting and long-term survival in Sweden. Acta Oncol 2011; 50: 1220–7.
Jung K, Park S, Kong H, Won Y, Boo Y, Shin H, et al. Cancer statistics in Korea: incidence, mortality and survival in 2006–2007. J Korean Med Sci 2010; 25: 1113–21.
Garrity AR, Morton GA, Morton JC . Nutraceutical mangosteen composition. Official gazette of the United States patent and trademark office patents 2004; 1282 (1US 6730333).
Nakatani K, Nakahata N, Arakawa T, Yasuda H, Ohizumi Y . Inhibition of cyclooxygenase and prostaglandin E-2 synthesis by gamma-mangostin, a xanthone derivative in mangosteen, in C6 rat glioma cells. Biochem Pharmacol 2002; 63: 73–9.
Matsumoto K, Akao Y, Kobayashi E, Ohguchi K, Ito T, Tanaka T, et al. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J Nat Prod 2003; 66: 1124–7.
Devi Sampath P, Vijayaraghavan K . Cardioprotective effect of alpha-mangostin, a xanthone derivative from mangosteen on tissue defense system against isoproterenol-induced myocardial infarction in rats. J Biochem Mol Toxicol 2007; 21: 336–9.
Sampath PD, Vijayaragavan K . Ameliorative prospective of alpha-mangostin, a xanthone derivative from Garcinia mangostana against beta-adrenergic cathecolamine-induced myocardial toxicity and anomalous cardiac TNF-alpha and COX-2 expressions in rats. Exp Toxicol Pathol 2008; 60: 357–64.
Nabandith V, Suzui M, Morioka T, Kaneshiro T, Kinjo T, Matsumoto K, et al. Inhibitory effects of crude alpha–mangostin, a xanthone derivative, on two different categories of colon preneoplastic lesions induced by 1,2-dimethylhydrazine in the rat. Asian Pac J Cancer Prev 2004; 5: 433–8.
Kang Y, Park M, Heo S, Park S, Kang KW, Park P, et al. The radio-sensitizing effect of xanthohumol is mediated by STAT3 and EGFR suppression in doxorubicin-resistant MCF-7 human breast cancer cells. Biochim Biophys Acta 2013; 1830: 2638–48.
Moon S, Kim D, Cha Y, Jeon I, Song J, Park K . PI3K/Akt and Stat3 signaling regulated by PTEN control of the cancer stem cell population, proliferation and senescence in a glioblastoma cell line. Int J Oncol 2013; 42: 921–8.
Jiang Q, Dai L, Cheng L, Chen X, Li Y, Zhang S, et al. Efficient inhibition of intraperitoneal ovarian cancer growth in nude mice by liposomal delivery of short hairpin RNA against STAT3. J Obstet Gynaecol 2013; 39: 701–9.
Acknowledgements
The authors thank the staff of the Institution of Genetic Disease Research at Xi'an Jiaotong University for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shan, T., Cui, Xj., Li, W. et al. α-Mangostin suppresses human gastric adenocarcinoma cells in vitro via blockade of Stat3 signaling pathway. Acta Pharmacol Sin 35, 1065–1073 (2014). https://doi.org/10.1038/aps.2014.43
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2014.43
Keywords
This article is cited by
-
Anticancer activity of dietary xanthone α-mangostin against hepatocellular carcinoma by inhibition of STAT3 signaling via stabilization of SHP1
Cell Death & Disease (2020)
-
Alpha-mangostin from mangosteen (Garcinia mangostana Linn.) pericarp extract reduces high fat-diet induced hepatic steatosis in rats by regulating mitochondria function and apoptosis
Nutrition & Metabolism (2016)
-
α-Mangostin-encapsulated PLGA nanoparticles inhibit pancreatic carcinogenesis by targeting cancer stem cells in human, and transgenic (KrasG12D, and KrasG12D/tp53R270H) mice
Scientific Reports (2016)