Abstract
Aim:
Sevoflurane postconditioning (SpostC) has been shown to protect the heart from ischemia-reperfusion (I/R) injury. In this study, we examined whether SpostC affected autophagic flux in myocardial tissues that contributed to its cardioprotective effects in rats following acute I/R injury.
Methods:
SD rats underwent 30 min of left anterior descending coronary artery ligation followed by 120 min of reperfusion. The rats were subjected to inhalation of 2.4% (v/v) sevoflurane during the first 5 min of reperfusion, and chloroquine (10 mg/kg, ip) was injected 1 h before I/R. Myocardial infarct size was estimated using TTC staining. Autophagosomes in myocardial tissues were detected under TEM. Expression of LC3B-II, beclin-1, p62/SQSTM1, cathepsin B, caspase-3 and cleaved PARP was assessed using Western blot analysis. Plasma cardiac troponin I was measured using ELISA. Cardiomyocyte apoptosis was evaluated with TUNEL staining.
Results:
I/R procedure produced severe myocardium infarct and apoptosis accompanied by markedly increased number of autophagosomes, as well as increased levels of LC3B-II, beclin-1 and p62 in myocardial tissues. SpostC significantly reduced infarct size, attenuated myocardial apoptosis, restored intact autophagic flux and improved the lysosomal function in myocardial tissues. Administration of chloroquine that blocked autophagic flux abrogated the cardioprotective effects of SpostC.
Conclusion:
SpostC exerts its cardioprotective effects in rats following I/R injury via restoring autophagic flux in myocardial tissues.
Similar content being viewed by others
Introduction
Methods to reduce the risk of cardiomyocyte death after reperfusion remain a challenge1. Pharmacologic interventions have been reported that can achieve a cardioprotective effect at the onset of reperfusion and that have the advantage of non-invasiveness and feasibility2. Studies have shown that volatile anesthetics reduce myocardial damage in clinical practice and experimental research3. Previous studies on sevoflurane postconditioning (SpostC) have furthered the understanding of the role of anesthetic postconditioning in cardioprotection4; however, the exact mechanisms responsible for the effects of SpostC are unclear.
Sevoflurane activates several pro-survival kinases, including protein kinase B (Akt)5, extracellular signal-regulated kinase (ERK) 1/26, and AMP-activated protein kinase (AMPK)4. These signals participate in regulating autophagy7,8. Autophagy provides a beneficial role against ischemic stress after the activation of AMPK9. Large amounts of reactive oxygen species (ROS) and damage to organelles occur during the first few minutes of reperfusion. Autophagy is involved in the degradation and removal of damaged organelles and proteins10. Based on these data, we aimed to determine how autophagy is involved in the effects of SpostC on ischemia-reperfusion (I/R) injury.
Cardioprotective interventions, such as caloric restriction11,12, exercise13, and chloramphenicol succinate14, have been reported to initiate autophagy. Ischemic preconditioning also induces autophagy to promote cardioprotective effects15,16. Housekeeping levels of autophagy are required for normal cardiac action; however, the autophagy response to stimuli is either an adaptive response or a detrimental effect9. Autophagy is a dynamic process, beginning with the induction of autophagosome formation and ending with autophagosome degradation in lysosomes17,18. Autophagic flux is the pathway from the formation of autophagosomes to autolysosome degradation, which is determined by measuring the number of autophagosomes or autophagic markers over time in the presence and absence of autophagosome-lysosome fusion and lysosomal degradation inhibitors19. Recent evidence suggests that impaired clearance and subsequent accumulation of autophagosomes leads to cell death in cardiac I/R injury20. Despite previous reports regarding how cardioprotective interventions affect autophagy, the mechanism by which autophagy, as a dynamic process, participates in the cardioprotective role of SpostC requires further elucidation. Moreover, the relationship between the cardioprotective role of SpostC and abnormal autophagic flux-induced reperfusion injury should be clarified.
We hypothesized that SpostC exposure during early reperfusion promotes autophagic flux. By blocking the degradative phase of autophagy with the lysosomotropic agent chloroquine (CQ), we determined whether the late stage of autophagic flux is related to the cardioprotective effects caused by SpostC and whether the increased clearance of autophagosomes conferred by SpostC has a cardioprotective effect on infarct size and apoptotic cell death.
Materials and methods
A total of 123 male Sprague–Dawley (8 weeks old, 250–300 g) rats were used in this study. Three rats were excluded for irreversible ventricular fibrillation, and one rat was excluded for loss of blood. This study was conducted according to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA). All experimental animals were approved by the Fuwai Hospital Animal Care and Use Committee (number 2012-5-60-GZ2011; Beijing, China).
Experimental protocol
To determine whether autophagy is involved in the mechanism of action of SpostC, rats were randomly assigned into one of seven groups (Figure 1). In group 1 (Sham), the rats underwent a time-matched procedure without ligation of the left anterior descending (LAD) artery and sevoflurane inhalation. In group 2 (Sham+SpostC), the rats underwent a time-matched sham procedure without ligation of the LAD, but were subjected to inhalation of sevoflurane at the matched time-point during the first 5 min of reperfusion. In group 3 (I/R), the rats underwent 30 min of LAD ligation and then either 30 or 120 min of reperfusion. In group 4 (I/R+SpostC), the rats received 2.4% (v/v) sevoflurane (Maruishi Pharmaceutical, Osaka, Japan) during the first 5 min of reperfusion. Chloroquine (CQ) is an autophagy inhibitor that blocks the degradative phase of autophagy. To determine whether the efficacy of SpostC was maintained with CQ (Sigma-Aldrich, St Louis, MO, USA) treatment, additional rats were administered intra-peritoneal injections of CQ (10 mg/kg) 1 h before surgical operation. Group 5 was the CQ+Sham group, in which the rats underwent a time-matched sham operation without ligation of the LAD and inhalation of sevoflurane. Group 6 was the CQ+I/R group, in which the rats underwent the same procedure as I/R group following CQ injection. The final group was the CQ+I/R+SpostC group, in which the rats received CQ and underwent the same procedure as the myocardial I/R protocol, but were given 2.4% (v/v) sevoflurane [1 min of aveolar concentration (1MAC)] during the first 5 min of reperfusion.
Experimental protocol. Rats were randomly assigned in seven groups (n=17). Chloroquine (upward dark triangle) or saline (upward white triangle) was injected 1 h before surgery. Rats underwent a procedure with ligation of the LAD for 30 min (dark bar), which was followed by 30 min (n=35) or 120 min (n=84) of reperfusion (open bar). Thirty-five rats underwent 30 min of LAD occlusion followed by 30 min of reperfusion (except for sham-operated rats). This protocol followed for the transmission electron microscopy (TEM) analysis (n=5), WB analysis (n=3), and cathepsin B activity assay (n=3). Forty-two rats were used for TTC staining, and another forty two rats were used for TUNEL staining after 120 min of reperfusion (n=6). Blood samples for the cTnI assay were collected after 120 min of reperfusion from the rats used in the TTC staining and TUNEL staining (n=7–13). Sixty-five blood samples were collected after 120 min of reperfusion. Sham-operated rats underwent a procedure without ligation of the LAD, but were time-matched. Sevoflurane was inhaled for 5 min immediately at the time of first reperfusion. LAD, left anterior descending coronary artery; I, ischemia; R, reperfusion; SpostC, sevoflurane postconditioning; CQ, chloroquine.
General preparations of animals
Our previous study described the preparation of animals in detail21. Here, we describe the protocol in brief. Rats were anesthetized with the intra-peritoneal injection of 10% chloral hydrate (300 mg/kg). Animals were intubated and ventilated. Mechanical ventilation was achieved with a positive-pressure ventilator for small animals (Model Inspira 557058, Harvard Apparatus, Holliston, MA, USA) using a tidal volume of 2–3 mL for each rat, 50% of inspired oxygen concentration, and a rate of 60–80 beats per min. Sevoflurane was delivered by a sevoflurane vaporizer (Dräger Vapor 2000, Drägerwerk AG & Co, Lübeck, Germany) attached to the ventilator. Sevoflurane was administered at a concentration of 2.4% (v/v) during the first 5 min of reperfusion, which was the same dose as used previously in vivo in rats22,23. The concentrations of O2, CO2, and sevoflurane in the expiratory gas were continuously measured using a volatile anesthetic gas monitor (Datex Capnomac Ultima, Division of Instrumentarium Corp, Helsinki, Finland). The respiratory rate was adjusted to maintain partial pressure of carbon dioxide within physiologic limits (end-tidal carbon dioxide, 35±5 mmHg). After exposure of the heart, a 6.0 silk ligation suture was looped around the LAD for subsequent occlusion. The ligature success of the LAD was judged by a color change at the area at risk (AAR), which was further confirmed by a QRS wave change during electrocardiography (ECG).
Ultrastructure examination
Thirty minutes after reperfusion, the rat hearts were removed. Two samples of fresh myocardial tissue (approximately 1 mm3 in size) were obtained 3 mm above the apex from the AAR of the left ventricle (LV). The tissues were fixed with 5% glutaraldehyde overnight at 4 °C, washed 3 times with phosphate-buffered saline, and fixed again with 1% osmium tetraoxide for 2 h. Ultra-thin sections were acquired by standard procedures. The sections were stained with uranyl acetate and lead citrate and then observed using a transmission electron microscope (JEM-1400; JEOL, Tokyo, Japan). Quantitative morphometric analysis of autophagic vacuoles was performed by a blinded observer. Five rats were assigned in each group. Ten fields were examined for each rat.
Western blotting
Thirty minutes after reperfusion, myocardial tissue was collected from the AAR of the LV. The tissue samples were immediately frozen in liquid nitrogen. Total protein was extracted with a protein extraction kit (Applygen Technologies, Beijing, China). The concentration of the total protein was detected with a BCA protein assay kit (Applygen Technologies). Eighty micrograms of total protein was separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were then transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% non-fat dry milk or 5% bovine serum albumin (BSA) for 1.5 h. After blocking, the membranes were rinsed three times with TBS-Tween (5 min each). The membranes were incubated overnight at 4 °C with primary antibodies against LC3B (1:500 dilution; Cell Signaling Technology, Beverly, MA, USA), beclin-1 (1:500 dilution; Cell Signaling Technology), p62 (1:500 dilution; Cell Signaling Technology), caspase-3 (1:500 dilution; Cell Signaling Technology), PARP (1:500 dilution; Cell Signaling Technology), and cathepsin B (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Peroxidase-conjugated affinipure goat anti-rabbit IgG [1:5000 dilution; Zhongshan Golden Bridge Biotechnology (ZSGB-BIO), Beijing, China] was used as a secondary antibody; β-actin (1:2000 dilution; ZSGB-BIO) was used as a protein loading control with peroxidase-conjugated affinipure goat anti-mouse IgG (1:5000 dilution; ZSGB-BIO) as a secondary antibody. Afterwards, the membranes were rinsed three times with TBS-Tween (5 min each). The membranes were incubated with secondary antibody for 2 h at room temperature and then three times with TBS-Tween (15 min each). The protein content was quantified using an enhanced chemiluminescent detection method with a Thermo ECL kit (SuperSignal, Thermo Scientific, Rockford, IL, USA) and a CCD camera running Quantity One software (Bio-Rad, Berkeley, CA, USA).
Lysosomal activity assay
Myocardial tissue samples were collected after 30 min of reperfusion from the AAR of the LV. The enzyme activity of cathepsin B was determined with a cathepsin B activity fluorometric assay kit (Biovision, Mountain View, CA, USA) according to the manufacturer's protocol. Briefly, the tissue samples were completely homogenized by a Dounce homogenizer. The issue lysates were centrifuged at 1.2×104 g for 12 min at 4 °C, and the supernatant was used for enzymatic assay and the measurement of protein concentration. The protein enzymatic assay was incubated at 37 °C for 1–2 h with 10 mmol/L Ac-RR-AFC (substrate for cathepsin B). After incubation for 1 h, the relative fluorescence units (RFUs) were measured by spectrofluorometric analyses by using a UV-fluorescent spectrophotometer (SFM25, Bio-Tek, Burlington, VT, USA) with excitation and emission settings of 400 and 505 nm, respectively.
Myocardial infarct size
At the end of 120 min reperfusion, the LAD coronary artery was reoccluded, and then, Evans blue (1%) was administered through the carotid vein to stain the normal region of the left ventricle (LV). The normal reperfusion area was stained in blue, while the area subjected to ischemia was cyanotic. The heart was excised rapidly and cut into five slices (1-mm-thick cross-section). The slices were incubated in a 0.1% solution of TTC (pH=7.4) for 15 min at 37 °C, and fixed in 4% paraformaldehyde for 24 h at room temperature. The infarct area was calculated by computer-based planimetry (Image J 1.43; National Institutes of Health, Bethesda, MD, USA). The values are expressed as a percentage of the AAR over the area of the LV (AAR/LV, %) and infarct area/AAR (%). The averaged result of 5 slices was calculated. The severity of myocardial infarction was determined by the averaged outcome.
Serum collection and measurement of cardiac troponin I (cTnI) levels
Serum samples were collected at the end of the 120 min reperfusion. The blood was centrifuged at 1.8×103 g for 12 min at 4 °C to measure the levels of cTnI using an ELISA kit (Life Diagnostics, West Chester, PA, USA). The assay was performed according to the manufacturer's protocol. The blood cTnI levels were measured with a spectrophotometer (Infinite F200; Tecan Group, Männedorf, Switzerland) and expressed as nanograms per liter of serum.
Terminal deoxyribonucleotidyl transferase (TdT)-mediated biotin-16-dUTP nick end labeling (TUNEL) assay
To study apoptotic cell death at the end of the 120 min reperfusion, the heart was removed and rinsed by syringe with saline. The heart was then fixed in 4% paraformaldehyde solution for 48 h. After preparation of paraffin-embedded sections (6 μm), TUNEL staining was performed using an in situ cell death detection kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's instructions. Apoptotic nuclei were identified using DAB staining. The samples were counterstained with hematoxylin prior to analysis by microscopy. The ratio of apoptotic cardiac myocytes was determined by dividing the number of TUNEL-positive nuclei by the total number of nuclei. Twenty different fields for each section were observed from the AAR at ×400 magnification with a Nikon Labophot 2 microscope (ECLIPSE 80i/90i; Nikon, Tokyo, Japan).
Statistical analysis
The data are presented as the mean±SD. GraphPad Prism 5.0 (GraphPad, San Diego, CA, USA) was used to analyze the data. The significant differences among multiple groups were analyzed by ANOVA followed by the Newman-Keuls multiple comparison test. P<0.05 was considered to be statistically significant.
Results
Effects of SpostC on autophagic vacuoles in rats subjected to sham operations and I/R injury
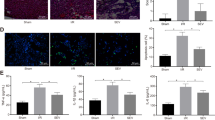
To determine whether autophagy is involved in the cardioprotection of SpostC, transmission electron microscopy (TEM) was used as a method for monitoring the changes in the number of autophagic vacuoles (AVs) after I/R injury and SpostC treatment. SpostC had an effect on autophagy formation, which was dependent on the type of stimulus (Figure 2A). There was a significant increase in the number of AVs with SpostC treatment in sham-operated rats (P<0.05, Sham group vs Sham+SpostC group; Figure 2B). In contrast, there was a significant decrease in the number of autophagosomes after SpostC treatment in rats subjected to I/R injury (P<0.05, I/R group vs I/R+SpostC group; Figure 2B), suggesting different effects of SpostC on autophagy.
Number of accumulated autophagic vacuoles in the hearts from rats subjected to sham-operation or I/R injury with SpostC treatment. (A) Representative transmission electron ultra-images showing autophagic vacuoles (×2500 magnification, bar=1 μm, denoted by arrows) in the following groups: Sham, Sham+SpostC, I/R, I/R+SpostC. An autophagic vacuole is characterized by a double membrane or one limiting membrane, which contain cytoplasmic material and/or organelles. (B) Quantitative analysis of the number of autophagic vacuoles in each group. Five rats were in each group, and ten fields were examined for each rat. The data are presented as the means±SD (n=5). An ANOVA followed by the Newman-Keuls test was used to determine statistical significance. bP<0.05 vs SpostC−+Sham. eP<0.05 vs SpostC−+I/R. Sham, rats in sham-operated groups; I/R, groups in which rats subjected to ischemia-reperfusion injury; SpostC, sevoflurane postconditioning.
Effect of SpostC on the expression of LC3B-II, beclin-1, p62, and cathepsin B and the activity of cathepsin B
Conversion of LC3B-I to LC3B-II (the phosphatidylethanolamine-conjugated form of LC3B-I) is an indicator of autophagosome formation. Beclin-1 dramatically increases and mediates the up-regulation of autophagy during reperfusion9. p62 is an adapter protein which links aggregated proteins sequestered in autophagosomes and promotes degradation in autolysosomes24. Thus, increased p62 is a reliable indicator of impaired autophagic flux, and activated autophagic flux is indicated by a decrease in p62 levels25. LC3B-II, beclin-1, and p62 are commonly used as autophagic markers. To evaluate the effects of SpostC on autophagy during reperfusion, tissue samples from the Sham, Sham+SpostC, I/R, and I/R+SpostC groups were used to detect the levels of expression of LC3B-II, beclin-1, and p62. The basal levels of LC3B-II, beclin-1 and p62 are shown in the sham control lysate (lane 1, Figure 3A). SpostC induced a decrease in p62 accumulation in the Sham group (lanes 1 and 2, respectively, Figure 3A; P<0.05, Figure 3D), suggesting that SpostC initiates an increase in the autophagic activity of Sham-operated rats. However, SpostC did not affect the levels of LC3B-II or beclin-1 in the rats in the Sham group (lane 1 and 2, respectively, Figure 3A; P>0.05, Figure 3B, 3C). The expression of LC3B-II, beclin-1, and p62 increased significantly in rat hearts subjected to I/R injury compared with the Sham group (lanes 1 and 3, respectively, Figure 3A; P<0.05, Figure 3B–3D), suggesting an increase in autophagic activity or impaired autophagic flux. However, the expression of LC3B-II, beclin-1, and p62 showed a significant decline in the I/R+SpostC group compared with the I/R group (lanes 3 and 4, respectively, Figure 3A; P<0.05, Figure 3B–3D), which indicated that SpostC suppressed the autophagic activity or accelerated the progress of autophagic flux by increasing the clearance of autophagosomes.
Expression levels of LC3B-II, p62, beclin-1, and cathepsin B and cathepsin B activity changes with SpostC treatment. (A) Representative western blots of LC3B-II, beclin-1, p62, and cathepsin B in the Sham, Sham+SpostC, I/R, and I/R+SpostC groups. The ratios of LC3B-II/LC3B-I (B), beclin-1/β-actin (C), p62/β-actin (D), and active cathepsin B/pro-cathepsin B (E) in the Sham group were defined as 1 in each blot, and the ratios of LC3B-II/LC3B-I, beclin-1/β-actin, p62/β-actin and active-cathepsin B/pro-cathepsin B in other groups in the same blot were normalized with the Sham to indicate levels of protein. (F) The relative fluorescence units (RFU) in the sham-operated rats were 3359.24, 3187.069, and 3100.487. The fold-increase in cathepsin B activity was determined by comparing the RFU with that of the sham-operated rats. The relative cathepsin B activities (compared to the Sham control group) were calculated and then plotted. Three rats in each group were examined. The data are presented as the means±SD (n=3). bP<0.05 vs Sham. eP<0.05 vs SpostC−+I/R. The abbreviations are defined as Figure 2.
Autophagosomes fuse with lysosomes and are then digested as an autolysosome. An accumulation of autophagosomes may cause the release of lysosomal proteases to the cytosol and eventually induce cell death26. Cathepsin B is one of the major lysosomal proteases, and thus, the level and redistribution of cathepsin B may influence lysosomal function27. Cathepsin B is required for autolysosome maturation28. Improvement of lysosomal function during reperfusion may ameliorate the fusion of autophagosomes and lysosomes, decreasing the accumulation of autophagosomes. To confirm that SpostC accelerates autophagic flux by promoting lysosomal function, thus leading to autophagosome degradation, we evaluated the levels and activities of cathepsin B. After I/R injury, there were elevated levels of active cathepsin B, as shown by immunoblot analysis (lanes 1 and 3, respectively, Figure 3A; P<0.05, Figure 3E). Cathepsin B activity was also increased after I/R injury (P<0.05, Figure 3F). SpostC improved lysosomal function after I/R injury, which was characterized by an increase in active cathepsin B (lanes 3 and 4, respectively, Figure 3A; P<0.05, Figure 3E) and in cathepsin B activity (P<0.05, Figure 3F).
Effect of SpostC on autophagic flux
An increase in the number of autophagic vacuoles after reperfusion can be due to an increase in autophagosome formation or attenuation in autophagosome clearance by lysosomes. To further determine whether the increase in the number of autophagic vacuoles after reperfusion is due to impaired autophagic flux, we used CQ as the major tool for measuring autophagic flux and to study the effects of SpostC on the autophagic dynamic changes of cardioprotection in vivo20. Our results demonstrated that the formation of autophagosomes was quite selective and associated with mitochondria (Figure 4A; arrowheads). Quantitative analysis of AVs showed that there was a significant increase in the number of AVs in sham-operated rats in the presence of CQ (1.0±1.2 in Sham group vs 5.2±1.3 in CQ+Sham group, P<0.05, Figure 4B). The number of AVs was not different in rats subjected to I/R injury in the presence or absence of CQ (18.8±2.6 in I/R group vs 20.0±3.2 in CQ+I/R group, P>0.05, Figure 4B). The effect of SpostC on the number of AVs in rats subjected to I/R injury was attenuated in the presence of CQ (11.2±1.3 in I/R+SpostC group vs 14.6±1.8 in CQ+I/R+SpostC group, P<0.05, Figure 4B). These results indicated that there was intact autophagic flux in sham-operated rats, impaired autophagic flux in rats subjected to I/R injury and an accelerated autophagic flux in rats treated with SpostC.
The number of accumulated autophagic vacuoles changes with chloroquine treatment. (A) Representative transmission electron ultra-images showing autophagic vacuoles (×2500 magnification, bar=1 μm, denoted by arrows) from the following six groups: Sham; I/R; I/R+SpostC; CQ+Sham; CQ+I/R; and CQ+I/R+SpostC. An autophagosome or initial autophagic vacuole (AVi) is characterized by a double membrane and contains mitochondria (Bar=200 nm, lower panel, left, Figure 4A). An autolysosome or late/degradative autophagic vacuole (AVd) is characterized by one limiting membrane, which contains partially degenerated cytoplasmic material and/or degenerated mitochondria (Bar=200 nm, lower panel, right, Figure 4A). (B) Quantitative analysis of the number of autophagic vacuoles in each group. Five rats were in each group, and ten fields were examined for each rat. The data are presented as the means±SD (n=5). An ANOVA followed by the Newman-Keuls test was used to determine statistical significance. bP<0.05 vs CQ−+Sham. eP<0.05 vs CQ++I/R. hP<0.05 vs I/R. kP<0.05 vs CQ−+I/R+SpostC. CQ, chloroquine; Sham, rats in sham-operated groups; I/R, groups in which rats subjected to ischemia reperfusion injury; SpostC, sevoflurane postconditioning. I/R+SpostC, groups in which rats subjected to ischemia reperfusion injury with sevoflurane postconditioning treatment.
Effect of SpostC with CQ treatment on the expression of LC3B-II, beclin-1, p62, and cathepsin B and the activity of cathepsin B
To further determine the effect of SpostC on autophagic flux, we evaluated the effect of CQ treatment on the expression of LC3B-II, beclin-1, p62, and cathepsin B and the activity of cathepsin B. The basal levels of LC3B-II, beclin-1, p62 and active-cathepsin B are shown in the sham control lysate (lane 1, Figure 5A). An increase in LC3B-II and p62 and a decrease in active cathepsin B in the presence of CQ in the sham-operated rats (lanes 1 and 4, respectively, Figure 5A; P<0.05, Figure 5B, 5D, 5E) implied an intact autophagic flux in the Sham group. Cardiac I/R injury induced a significant increase in the expression of LC3B-II, beclin-1, p62, and active cathepsin B in the I/R group compared with the Sham group (lanes 1 and 2, respectively, Figure 5A; P<0.05, Figure 5B–5E). p62 is digested after autophagosome fusion with lysosomes, which indicates autophagic flux. Thus, reperfusion injury induced an increase in LC3B-II accompanied with p62 accumulation (lanes 1 and 2, respectively, Figure 5A; P<0.05, Figure 5B, 5D), suggesting an attenuated clearance of autophagosomes. Moreover, in the presence of CQ, no additional changes in LC3B-II, beclin-1, p62, and active-cathepsin B were observed in the CQ+I/R group compared with the I/R group (lanes 2 and 5, respectively, Figure 5A; P>0.05, Figure 5B–5E), which can be explained by impaired autophagosome clearance induced by reperfusion injury. SpostC decreased the levels of LC3B-II, beclin-1, p62 and increased the level of active cathepsin B in the I/R+SpostC group compared with the I/R group (lanes 2 and 3, respectively, Figure 5A; P<0.05, Figure 5B–5E). SpostC decreased the levels of LC3B-II and p62 and increased the level of active cathepsin B in the CQ+I/R+SpostC group compared with the CQ+I/R group (lanes 5 and 6, respectively, Figure 5A; P<0.05, Figure 5B, 5D, 5E), suggesting that SpostC accelerated the autophagic flux by increasing the clearance of autophagosomes. Moreover, CQ attenuated the effect of sevoflurane on the reduction of p62 expression in the CQ+I/R+SpostC group compared with the I/R+SpostC group (lanes 3 and 6, respectively, Figure 5A; P<0.05, Figure 5D), suggesting that the effects of SpostC on autophagic activity is at least partly dependent on the increased clearance of impaired autophagosomes. These results were further supported by the change in cathepsin B activity with CQ treatment (Figure 5F).
Expression levels of LC3B-II, beclin-1, and p62, and cathepsin B and cathepsin B activity change with chloroquine treatment. (A) Representative Western blotting bands of LC3B-II, beclin-1, p62, and cathepsin B in the Sham, I/R, and I/R+SpostC groups in the presence (+) or absence (–) of CQ treatment. (B, C, D, E) The ratios of LC3B-II/LC3B-I, beclin-1/β-actin, p62/β-actin, and active-cathepsin B/pro-cathepsin B in the Sham group were defined as 1 in each blot, and the ratios of LC3B-II/LC3B-I, beclin-1/β-actin, p62/β-actin, and active-cathepsin B/pro-cathepsin B in other groups in the same blot were normalized with the Sham group to indicate the levels of protein. (F) The relative fluorescence units (RFUs) in the sham-operated rats were 3293.398, 3115.282, and 3673.434. The fold-increase in cathepsin B activity was determined by comparing the RFU with that of the sham-operated rats. The relative cathepsin B activities (compared with the Sham group) were calculated and then plotted. The data are presented as the means±SD (n=3, three rats were in each group). bP<0.05 vs CQ−+Sham. dP>0.05 vs CQ−+I/R. hP<0.05 vs I/R. kP<0.05 vs CQ−+I/R+SpostC. The abbreviations are defined as in Figure 4.
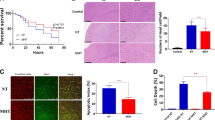
Cardioprotective effect of SpostC was abolished by CQ
The infarct size was 44.2%±3.1% of the AAR in the I/R group. SpostC reduced the infarct size to 21.1%±4.7% in the I/R+SpostC group (P<0.05, Figure 6B). Administration of the autophagic inhibitor CQ did not increase infarct size in the CQ+I/R group [44.2%±3.1% (I/R group) vs 45.3%±4.0% (CQ+I/R group); P>0.05, Figure 6B]. CQ administration eliminated the cardioprotection elicited by sevoflurane [infarct size, 21.1%±4.7% (I/R+SpostC group) vs 39.5±3.9% (CQ+I/R+SpostC group); P<0.05, Figure 6B]. Additionally, the SpostC decreased the infarct size in the CQ+I/R+SpostC group compared with the CQ+I/R group [39.5%±3.9% (CQ+I/R+SpostC group) vs 45.3%±4.0% (CQ+I/R group); P<0.05, Figure 6B]. Myocardial infarctions were not observed in the Sham and CQ+Sham groups (Figure 6B). There were no significant differences in AAR/LV among the groups [52.9%±8.0% (Sham group), 54.5%±3.0% (I/R group), 53.6%±5.4% (I/R+SpostC group), 56.2%±4.9% (CQ+Sham group), 54.9%±3.8% (CQ+I/R group), and 54.0%±9.4% (CQ+I/R+SpostC group); P>0.05, Figure 6C].
Chloroquine abolished the cardioprotective effect of SpostC. (A) Five representative slices of the left ventricle (LV) stained by Evans blue and TTC from 6 rats in Sham (1), I/R (2), I/R+SpostC (3), CQ+Sham (4), CQ+I/R (5), and CQ+I/R+SpostC (6) groups. Heart slices were incubated in TTC. The infarcted myocardium within the area at risk (AAR) was not stained and became pale, and the viable myocardium was stained red. (B) Infarct size, expressed as a percentage of the myocardium at risk for Sham, I/R, I/R+SpostC, CQ+Sham, CQ+I/R, and CQ+I/R+SpostC groups. (C) Quantitative analysis of AAR/LV in Sham, I/R, I/R+SpostC, CQ+Sham, CQ+I/R, and CQ+I/R+SpostC groups. (D) Cardiac troponin I levels from Sham, I/R, I/R+SpostC, CQ+Sham, CQ+I/R, and CQ+I/R+SpostC groups. Troponin I is presented in ng/L. The data are presented as the means±SD (n=7–13). bP<0.05 vs Sham. eP<0.05 vs I/R. hP<0.05 vs CQ−+I/R+SpostC. The abbreviations are defined as in Figure 4.
After 120 min of reperfusion, the plasma cTnI activity was 11.9±3.1 ng/L in the Sham group and 15.2±2.6 ng/L in the CQ+Sham group (P>0.05, Figure 6D), 28.7±4.2 ng/L in the I/R group and 28.0±2.8 ng/L in the CQ+I/R group (P>0.05, Figure 6D), 19.3±2.6 ng/L in the I/R+SpostC group and 23.4±2.3 ng/L in the CQ+ I/R+SpostC group (P<0.05, Figure 6D).
CQ abolished the effects of SpostC on cardiac apoptosis
After 120 min of reperfusion, there were no TUNEL-positive cells in the Sham group (Figure 7B). The percentage of TUNEL-positive cells was 4.7±2.1 in the CQ+Sham group (P<0.05 vs the Sham group, Figure 7B), 25.8±3.8 in the I/R group and 28.0±2.7 in the CQ+I/R group (P>0.05, Figure 7B), 13.6±4.2 in the I/R+SpostC group and 23.4±3.0 in the CQ+ I/R+SpostC group (P<0.05, Figure 7B).
Myocardial apoptosis increased after CQ treatment. (A) Representative images of TUNEL staining from 6 rats in Sham, I/R, I/R+SpostC, CQ+Sham, CQ+I/R, and CQ+I/R+SpostC groups. TUNEL-positive nuclei of cardiomyocytes are stained brown (denoted by arrows, bar=50 μm). (B) TUNEL-positive cells, expressed as a percentage of the total cardiomyocytes for Sham, I/R, I/R+SpostC, CQ+Sham, CQ+I/R, and CQ+I/R+SpostC groups. (C) Representative Western blots of cleaved caspase-3/ procaspase-3, and cleaved PARP in the Sham, I/R, and I/R+SpostC groups in the presence (+) or absence (–) of CQ. (D, E) The ratios of cleaved caspase-3/procaspase-3, and cleaved PARP/β-actin in the Sham group were defined as 1 in each blot, and the ratios of cleaved caspase-3/procaspase-3, and cleaved PARP/β-actin in other groups in the same blot were normalized with the Sham group to indicate the levels of protein. The data are presented as the means±SD (n=3, three rats in each group). bP<0.05 vs Sham. eP<0.05 vs I/R. hP<0.05 vs CQ−+I/R+SpostC. The abbreviations are defined as in Figure 4.
To further investigate that apoptosis was involved in the cardioprotective effects of SpostC, caspase-3 and cleaved PARP were used for the detection of apoptosis after 30 min of reperfusion (Figure 7C). SpostC attenuated the levels of cleaved caspase-3 and PARP (P<0.05, Figure 7D, 7E). The levels of cleaved caspase-3 and PARP increased in the presence of CQ (P<0.05, Figure 7D, 7E). These results indicated that CQ attenuated the effects of SpostC on apoptosis.
Discussion
In this study, we showed that the effect of SpostC against I/R injury was mediated by restoring intact autophagic flux, which was characterized by a decline in autophagosome accumulation and attenuated autophagic markers, and improved lysosomal function during the reperfusion period.
A block in autophagic flux that results in autophagosome accumulation must be differentiated from stimuli that result in increased autophagic activity. During reperfusion, autophagosomes accumulate because of a block in trafficking to lysosomes, whereas an increase in autophagic vacuoles (AVs) may reflect a reduction in degradative activity. In this study, the number of autophagic vacuoles in the hearts subjected to I/R injury did not change in the presence of CQ, as determined by electron microscopy, which supported the conclusion that the impaired autophagic flux was due to a decrease in autophagosome clearance during reperfusion. The enhancement of autophagic flux by SpostC was detected by a decreased accumulation of autophagosomes as well as a decrease in the LC3B-II and p62 levels in the rats subjected to I/R injury. This elevation of autophagic flux by SpostC during I/R injury differed from the activation of autophagy by SpostC in sham-operated rats. We found that SpostC in sham-operated rats led to an increase in AVs and a decrease in p62, which could indicate accelerated autophagic flux, both at early and late stages of the process. An increase in LC3B-II with p62 accumulation suggests impaired autophagosome clearance during reperfusion29. Recently, several studies have reported that reperfusion injury induces impaired autophagic flux and excessive autophagy induces cell death20,30. After SpostC treatment, a decrease in p62 implies activated autophagic activity. However, if SpostC activates autophagy without the increased removal of autophagosomes, more autophagosomes would be blocked during reperfusion, which may induce detrimental effects of autophagy during reperfusion. Moreover, CQ abolished the SpostC effect on autophagic flux (increased AVs and p62 in the CQ+I/R+SpostC group compared with the I/R+SpostC, Figure 4B, 5D). These results may also explain the controversy that SpostC not only activates autophagy but also increases the removal of autophagosomes during I/R injury.
The present study showed that SpostC restored intact autophagic flux during reperfusion, which was characterized by a reduction of autophagosomes during reperfusion. Autophagosome clearance is the late stage of autophagic flux. Impaired autophagic flux at the late stage is closely related with lysosomal dysfunction. A previous study demonstrated that lysosomal function was activated in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion31. In this study, SpostC activated lysosomal function in the autophagy process, which was characterized by a decrease in p62 levels as well as increased expression and activation of cathepsin B (Figure 3D–3F and Figure 5D–5F). The mechanism responsible for the SpostC-dependent removal of autophagosomes may be related to the improvement of lysosomal function. However, how SpostC regulates autophagic flux through lysosomal function needs further elucidation.
Our previous study demonstrated that SpostC reduces ROS levels (reactive oxygen species) and attenuates mitochondrial injury32,33. Recently, a study has reported that the autophagosome accumulation induced by CQ increases ROS generation with the loss of mitochondrial membrane potential20. Autophagy can reduce ROS production by the removal of damaged mitochondria. ROS can regulate autophagy through distinct mechanisms based on distinctive cell types and stimulation conditions34. In the present study, increased autophagic flux by SpostC was characterized by an increase in the removal of autophagosomes during reperfusion. Whether SpostC reduces ROS and attenuates mitochondrial injury through the direct regulation of autophagy remains unclear. Conversely, the mechanism responsible for the effects of SpostC on autophagic activity during I/R injury may be related to the regulation of ROS or other upstream signaling pathway.
It has been reported that delayed sevoflurane preconditioning confers cardioprotection against I/R injury via up-regulation of autophagy prior to ischemia35. This result is consistent with those of the present study; SpostC accelerated autophagic flux, characterized by an increase in LC3B-II (Figure 3B), in sham-operated rats. Intervention is different in delayed sevoflurane preconditioning and sevoflurane postconditioning. Thus, the different intervention strategy of sevoflurane may have an influence on the mechanism against I/R injury. A previous study showed that ischemic preconditioning induces autophagy formation and initiates cardioprotective effects against I/R injuries15. It has also been reported that pharmacological pretreatment with the adenosine A1 receptor selective agonist CCPA induces autophagy and confers cardioprotection against I/R injury36. In this previous study, the preconditioning was performed prior to the ischemic period and less damage occurred during the ischemic period36. After CCPA pre-treatment, diminished autophagy during simulated ischemia and reperfusion occurred because the cells experienced less stress, and less autophagy occurred as a response to reperfusion injury. Postconditioning was applied at the time of reperfusion, with no effects on autophagy during the ischemic time. Preconditioning with CCPA or postconditioning with sevoflurane and the resulting decline in the number of autophagosomes conferred beneficial effects during reperfusion.
The role of autophagy in I/R injury is controversial37,38. In cardiac acute I/R injury, autophagy mediated by AMP-activated protein kinase during ischemia is beneficial, whereas beclin-1-mediated autophagy during reperfusion is detrimental9. The infarct size of ischemic hearts after I/R is reduced in beclin-1+/− mice, an effect assumed to be due to diminished autophagy during reperfusion39. In contrast, Hamacher-Brady et al40 reported that autophagic flux is impaired at induction and degradation and that overexpression of beclin-1 is protective against I/R injury. How to interpret these discrepancies about the role of autophagy in I/R injury is not straightforward. Beclin-1 participates in various physical and pathologic processes using the BH3 domain. The interaction between beclin-1 and other proteins may confer protective effects independent of autophagy41. Therefore, other compensatory pathways may be up-regulated in beclin-1+/− mice.
Autophagy and apoptosis are two well-controlled processes, and several common pathways contribute to the regulation of both autophagy and apoptosis in cell death42. A previous study demonstrated that apoptosis occurs in autophagy-induced cardiac myocytes43. Autophagy has been shown to engage in a complex interplay with apoptosis. Recently, several studies have reported interconnections between the apoptotic and the autophagic pathways, as well as their alternative functions in cell survival or death. For example, treatment with autophagic inhibitors induces programmed cell death in cardiomyocytes20. ATG7 depletion promotes apoptosis44. In the present study, CQ (an autophagic flux inhibitor) abolished the anti-apoptotic effects of SpostC. In addition, inhibition of autophagic flux promoted the activation of caspase-3 and PARP. Therefore, SpostC accelerates autophagic flux and inhibits cardiomyocyte apoptosis. However, further studies will be needed to elucidate the interactions between the molecular mechanisms responsible for autophagic flux and apoptosis.
Currently, methods to measure autophagic flux in vivo are limited. We calculated autophagic flux in the presence or absence of CQ. CQ is a non-specific inhibitor of autophagy that has multiple effects on cell survival19. The dosage and intervention of CQ used in this study were based on a previous study20. However, the evaluation of autophagosomes by TEM yields a static picture at one point in time. Therefore, it is difficult to widely interpret the results in light of the dynamically changing process of autophagy.
Taken together, we showed that the cardioprotection caused by sevoflurane was characterized by attenuation of the accumulation of autophagosomes during reperfusion in rat hearts. However, additional studies are necessary to elucidate the signaling pathways that contribute to autophagy in SpostC. Whether interventions that directly target autophagy represent new therapeutic approaches in the treatment of I/R injuries remains to be determined.
Author contribution
Yu-lin ZHANG, Yun-tai YAO, Neng-xin FANG, and Jun-song GONG were participated in conceiving and designing the experiments; Yu-lin ZHANG, and Chen-hui ZHOU conducted the experiments; Yu-lin ZHANG, Yun-tai YAO, Neng-xin FANG, and Li-huan LI were involved with interpretation of the studies, analysis of the data and review of the manuscript; Yu-lin ZHANG and Yun-tai YAO wrote the paper.
References
Hausenloy DJ, Yellon DM . Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest 2013; 123: 92–100.
Eltzschig HK, Eckle T . Ischemia and reperfusion-from mechanism to translation. Nat Med 2011; 17: 1391–401.
De Hert SG, Turani F, Mathur S, Stowe DF . Cardioprotection with volatile anesthetics: mechanisms and clinical implications. Anesth Analg 2005; 100: 1584–93.
Lamberts RR, Onderwater G, Hamdani N, Vreden MJ, Steenhuisen J, Eringa EC, et al. Reactive oxygen species-induced stimulation of 5′AMP-activated protein kinase mediates sevoflurane-induced cardioprotection. Circulation 2009; 120: S10–15.
Zhu L, Lemoine S, Babatasi G, Lepage O, Massetti M, Gerard JL, et al. Sevoflurane- and desflurane-induced human myocardial post-conditioning through phosphatidylinositol-3-kinase/Akt signalling. Acta Anaesthesiol Scand 2009; 53: 949–56.
Chen HT, Yang CX, Li H, Zhang CJ, Wen XJ, Zhou J, et al. Cardioprotection of sevoflurane postconditioning by activating extracellular signal-regulated kinase 1/2 in isolated rat hearts. Acta Pharmacol Sin 2008; 29: 931–41.
Aviv Y, Shaw J, Gang H, Kirshenbaum LA . Regulation of autophagy in the heart: you only live twice. Antioxid Redox Signal 2011; 14: 2245–50.
Fimia GM, Piacentini M . Regulation of autophagy in mammals and its interplay with apoptosis. Cell Mol Life Sci 2010; 67: 1581–88.
Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and beclin 1 in mediating autophagy. Circ Res 2007; 100: 914–22.
Gottlieb RA, Finley KD, Mentzer RM . Cardioprotection requires taking out the trash. Basic Res Cardiol 2009; 104: 169–80.
Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R . Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev 2012; 11: 390–8.
Han X, Ren J . Caloric restriction and heart function: Is there a sensible link? Acta Pharmacol Sin 2010; 31: 1111–7.
Chen CY, Hsu HC, Lee BC, Lin HJ, Chen YH, Huang HC, et al. Exercise training improves cardiac function in infarcted rabbits: Involvement of autophagic function and fatty acid utilization. Eur J Heart Fail 2010; 12: 323–30.
Sala-Mercado JA, Wider J, Undyala VV, Jahania S, Yoo W, Mentzer RM, et al. Profound cardioprotection with chloramphenicol succinate in the swine model of myocardial ischemia-reperfusion injury. Circulation 2010; 122: S179–84.
Huang C, Yitzhaki S, Perry CN, Liu W, Giricz Z, Mentzer RM, et al. Autophagy induced by ischemic preconditioning is essential for cardioprotection. J Cardiovasc Transl Res 2010; 3: 365–73.
Yan WJ, Dong HL, Xiong LZ . The protective roles of autophagy in ischemic preconditioning. Acta Pharmacol Sin 2013; 34: 636–43.
Choi AM, Ryter SW, Levine B . Autophagy in human health and disease. N Engl J Med 2013; 368: 651–62.
Zhang XJ, Chen S, Huang KX, Le WD . Why should autophagic flux be assessed? Acta Pharmacol Sin 2013; 34: 595–99.
Iwai-Kanai E, Yuan H, Huang C, Sayen MR, Perry-Garza CN, Kim L, et al. A method to measure cardiac autophagic flux in vivo. Autophagy 2008; 4: 322–9.
Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Hill JA, et al. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 2012; 125: 3170–81.
Li H, Zhou C, Chen D, Fang N, Yao Y, Li L . Failure to protect against myocardial ischemia-reperfusion injury with sevoflurane postconditioning in old rats in vivo. Acta Anaesthesiol Scand 2013; 57: 1024–31.
Obal D, Preckel B, Scharbatke H, Mullenheim J, Hoterkes F, Thamer V, et al. One MAC of sevoflurane provides protection against reperfusion injury in the rat heart in vivo. Br J Anaesth 2001; 87: 905–11.
Obal D, Scharbatke H, Barthel H, Preckel B, Mullenheim J, Schlack W . Cardioprotection against reperfusion injury is maximal with only two minutes of sevoflurane administration in rats. Can J Anaesth 2003; 50: 940–5.
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. P62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 2007; 282: 24131–45.
Bjorkoy G, Lamark T, Pankiv S, Overvatn A, Brech A, Johansen T . Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol 2009; 452: 181–97.
Gottlieb RA, Mentzer RM . Autophagy during cardiac stress: Joys and frustrations of autophagy. Annu Rev Physiol 2010; 72: 45–59.
Appelqvist H, Waster P, Kagedal K, Ollinger K . The lysosome: from waste bag to potential therapeutic target. J Mol Cell Biol 2013; 5: 214–26.
Koike M, Shibata M, Waguri S, Yoshimura K, Tanida I, Kominami E, et al. Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses (Battendisease). Am J Pathol 2005; 167: 1713–28.
Mizushima N, Yoshimori T . How to interpret LC3 immunoblotting. Autophagy 2007; 3: 542–5.
Jang BG, Choi BY, Kim JH, Kim MJ, Sohn M, Suh SW . Impairment of autophagic flux promotes glucose reperfusion-induced neuro2a cell death after glucose deprivation. PloS One 2013; 8: e76466.
Zhou J, Tan SH, Nicolas V, Bauvy C, Yang ND, Zhang J, et al. Activation of lysosomal function in the course of autophagy via mTOR1 suppression and autophagosome-lysosome fusion. Cell Res 2013; 23: 508–23.
Gong JS, Yao YT, Fang NX, Li LH . Sevoflurane postconditioning attenuates reperfusion-induced ventricular arrhythmias in isolated rat hearts exposed to ischemia/reperfusion injury. Mol Biol Rep 2012; 39: 6417–25.
Fang NX, Yao YT, Shi CX, Li LH . Attenuation of ischemia-reperfusion injury by sevoflurane postconditioning involves protein kinase B and glycogen synthase kinase 3 beta activation in isolated rat hearts. Mol Biol Rep 2010; 37: 3763–9.
Huang J, Lam GY, Brumell JH . Autophagy signaling through reactive oxygen species. Antioxid Redox Signal 2011; 14: 2215–31.
Qiao S, Xie H, Wang C, Wu X, Liu H, Liu C . Delayed anesthetic preconditioning protects against myocardial infarction via activation of nuclear factor-κB and upregulation of autophagy. J Anesth 2013; 27: 251–60.
Yitzhaki S, Huang C, Liu W, Lee Y, Gustafsson AB, Mentzer RM, et al. Autophagy is required for preconditioning by the adenosine A1 receptor-selective agonist CCPA. Basic Res Cardiol 2009; 104: 157–67.
Przyklenk K, Dong Y, Undyala VV, Whittaker P . Autophagy as a therapeutic target for ischaemia reperfusion injury? Concepts, controversies, and challenges. Cardiovasc Res 2012; 94: 197–205.
Gottlieb RA, Mentzer RM . Autophagy: an affair of the heart. Heart Fail Rev 2013; 18: 575–84.
Hariharan N, Zhai P, Sadoshima J . Oxidative stress stimulates autophagic flux during ischemia/reperfusion. Antioxid Redox Signal 2011; 14: 2179–90.
Hamacher-Brady A, Brady NR, Gottlieb RA . Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem 2006; 281: 29776–87.
Kang R, Zeh HJ, Lotze MT, Tang D . The beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 2011; 18: 571–80.
Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A . Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ 2009; 16: 966–75.
Yan WJ, Dong HL, Xiong LZ . The protective roles of autophagy in ischemic preconditioning. Acta Pharmacol Sin 2013; 34: 636–43.
Yue W, Hamai A, Tonelli G, Bauvy C, Nicolas V, Tharinger H, et al. Inhibition of the autophagic flux by salinomycin in breast cancer stem-like/progenitor cells interferes with their maintenance. Autophagy 2013; 9: 714–29.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No 81070098, Grant No 81200109).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Yl., Yao, Yt., Fang, Nx. et al. Restoration of autophagic flux in myocardial tissues is required for cardioprotection of sevoflurane postconditioning in rats. Acta Pharmacol Sin 35, 758–769 (2014). https://doi.org/10.1038/aps.2014.20
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2014.20
Keywords
This article is cited by
-
Regulation of autophagy of the heart in ischemia and reperfusion
Apoptosis (2023)
-
The neuroprotective mechanism of sevoflurane in rats with traumatic brain injury via FGF2
Journal of Neuroinflammation (2022)
-
Role of autophagy in regulation of cancer cell death/apoptosis during anti-cancer therapy: focus on autophagy flux blockade
Archives of Pharmacal Research (2020)
-
Sevoflurane postconditioning protects against myocardial ischemia/reperfusion injury by restoring autophagic flux via an NO-dependent mechanism
Acta Pharmacologica Sinica (2019)
-
New and revisited approaches to preserving the reperfused myocardium
Nature Reviews Cardiology (2017)