Abstract
Aim:
A novel hemostatic sealant based on the in situ gel formation of gelatin catalyzed by thrombin and crosslinked by transglutaminase was designed. The aim of this study was to investigate the efficacy of the hemostatic sealant in control of bleeding caused by liver trauma in dogs.
Methods:
Hepatic trauma that mimicked the grade III–IV rupture of liver was made in 20 dogs. The traumatic lesion was topically administered the hemostatic sealant (treatment group, n=10), or a thrombin solution (control group, n=10). The time to achieve hemostasis and the blood loss were measured. Contrast-enhanced ultrasound (CEUS) examination was performed in each animal on d 3, d 7, and d 10 d postoperatively to study the healing of the lesions.
Results:
The mean time to achieve hemostasis in the treatment group was significantly shorter than that in the control group (1.20±0.33 vs 6.70±0.64 min, P<0.05). The mean blood loss in the treatment group was significantly less than that in the control group (47.22±8.61 vs 79.29±11.97 mL, P<0.05). In CEUS examination, the traumatic lesions in the treatment group became significantly smaller on d 3, and disappeared on d 7, whereas the lesions in the control group still existed on d 10. Ascites were never found during 10 d follow-up in the treatment group but were observed on d 3 and d 7 in the control group.
Conclusion:
Compared with thrombin, the novel hemostatic sealant shows much better efficacy in hemostasis and may promote wound healing in dog liver trauma.
Similar content being viewed by others
Introduction
Hemorrhage remains the leading cause of abdominal wounds1, and thus, the quick and effective control of bleeding could greatly increase the rate of survival. Although conventional methods, such as suturing and cautery, are effective in terminating hemorrhage, limitations exist in their hepatic application due to the crisp texture and inaccessible location of the liver2. Topical hemostats, as a novel method, may contribute to coping with the issue of hemostasis when traditional surgical methods are ineffective or inappropriate3.
FloSeal™, a topical agent, is a hemostatic sealant composed of thrombin and a gelatin matrix derived from bovine collagen, which is crosslinked by glutaraldehyde. FloSeal has been certified to have a significant hemostatic efficacy in a range of surgical situations, including cardiac4, vascular5 and spinal surgeries6, liver rupture and resection7,8,9, renal injury10, partial nephrectomy (open and laparoscopic) and percutaneous nephrolithotomy11,12, endoscopic sinus surgery13 and gynecological surgery14,15. However, it is noteworthy that for in situ applications, there are safety concerns associated with the employment of glutaraldehyde16. Slight toxicity has been demonstrated in oral toxicology studies (eg, contact dermatitis when applied on the skin17). Hence, to serve as a non-toxic alternative to the chemical crosslinker glutaraldehyde, the calcium-dependent transglutaminase (TG) is investigated.
Initial studies with TG were aimed at its applications in the food industry18,19,20, and later studies laid more emphasis on its potential medical applications. It was proved to have an effect of enhancing the integrative bonding of articular cartilage wounds21, promoting the healing of skin wounds22, and serving in fibrin polymerization during blood clotting23. Additionally, TG was found to be effective in the crosslinking of gelatin18,24,25,26, and previous studies have shown that the gelatin-TG adhesive could achieve complete hemostasis in 2.5 min on liver wounds and femoral artery wounds in a murine model27.
A hemostatic sealant based on the in situ gel formation of gelatin catalyzed by thrombin and crosslinked by TG was investigated and discussed in this study. We conducted a randomized controlled study in a canine model of hepatic trauma to investigate the efficacy of this novel hemostatic sealant that was applied under no pressure.
Materials and methods
Preparation of hemostatic agents
Gelatin (180 Bloomg, type B, derived from bovine bones) was obtained from the Beijing University of Chemical Technology (National Gelatin Testing Center, Beijing, China). TG was obtained from Vickers Industrial Co, Ltd (Shanghai, China). Lyophilizing thrombin powder (500 UI) was obtained from Beijing First Biochemical Pharmaceutical Co, Ltd (Beijing, China). The calcium chloride injection was obtained from Shanghai Pharmaceutical Co, Ltd (Shanghai, China). Normal saline was provided by the Chinese People's Liberation Army General Hospital.
The gelatin solution (20% w/w) in PBS buffer (pH=7.4) was freshly prepared each day and maintained at 40 °C for no more than 1 h prior to use. TG dissolved in calcium chloride solution at a concentration of 10% (w/v) was prepared and stored on ice until use. The thrombin solution was prepared by mixing the calcium chloride injection and lyophilized thrombin powder. Immediately before applying, the novel hemostatic sealant was prepared by evenly mixing the warm gelatin solution (40 °C) with the thrombin and TG solutions at a 7:2:1 volume ratio.
The thrombin solution applied in the control group was prepared as described above; the concentration was 2000 UI of thrombin dissolved in 2 mL of solution.
Animals and anesthesia
All experiments were conducted in accordance with our institutional animal care and use committee protocols and the National Institute of Health guidelines for the care of laboratory animals (license number, SCXK [Beijing] 2007–0004). In this study, a total of 20 male hybrid dogs (aged 2–3 years old and weighing 15–19 kg) with healthy certificates were adopted after a 3 d acclimation period. Food was withheld for 18 h prior to surgery. All dogs received an antibiotic (benzylpenicillin sodium) intramuscularly for 10 d postoperatively.
General anesthesia was induced by an intravenous injection of pentobarbital sodium (3%, 30 mg/kg). Mechanical ventilation (System main unit model DC-120H, Nihon Kohden Corporation, Japan) was set with an initial tidal volume of 300 mL (15–19 kg) and a peak pressure of 6.5 cmH2O. The respiratory rate was set at 18 per minute. Catheters were placed in the femoral artery for measuring blood pressure and in the accessory cephalic vein for fluid resuscitation, anesthetic infusion and blood sample removal. The mean arterial pressure (MAP) was monitored continuously by the Microprocessor Ventilator 7200 series (Puritan Bennett Corporation, USA). Intravenous lactated Ringer's solution was administered to maintain the MAP at no less than 60 mmHg. Each dog was infused with approximately 500 mL of lactated Ringer's solution.
Surgical procedure
An abdominal midline incision was made, and the liver was exposed. According to the Organ Scaling Committee of the American Association for the Surgery of Trauma28 and combined with animal weight, an injury (4.0 cm in length, 4.0 cm in width and 2.0 cm in depth) was uniformly created using hemostatic clamp in the left lateral lobe of the liver to mimic grade III–IV rupture of liver. Then, they were randomly assigned to receive 2 mL of the topical hemostatic agent described above (treatment group, n=10) or the thrombin solution (control group, n=10). The time to achieve hemostasis and the weight of blood loss were recorded. The random assignment number was generated by a computer. To achieve the maximum hemostatic efficacy, the rapid application of the injection was begun at the deepest part of the traumatic lesion directly and was continued until the whole defect was completely filled with the injection. During the course of hemostasis, there was no additional compression. All blood was wiped from the abdominal cavity with gauze, and the abdomen was closed.
The time to achieve hemostasis was defined as the time from the moment after the injection to the absence of any apparent residual bleeding from the lesion site. The weight of blood loss was calculated by subtracting the weight of the gauze before surgery from the weight of the gauze after all visible blood and blood clots were wiped away.
CEUS evaluation
Conventional ultrasound and CEUS were performed using a CX50 system (Philips Medical Systems, Andover, MA, USA). A L12-3 transducer with 3.0–12 MHz was used. The CEUS employed a pulse inversion harmonic and energy modulated technique at a low acoustic power (a mechanical index of 0.07), which detects not only the non-linear second harmonic response of microbubbles but also the strong non-linear fundamental component, thus increasing the signal-to-noise ratio by 15–20 dB and providing a much stronger contrast signal. The scan settings during this experiment (including the gain, scanning depth and time gain control) were optimized for each region independently. The focus was set to the deeper aspect of the lesion being examined.
The ultrasound contrast agent used in this study was SonoVue (Bracco), a suspension of stabilized microbubbles of inert gas (sulfur hexafluoride) covered by a phospholipid membrane29. The agent was supplied as lyophilized product and was reconstituted by mixing with 5.0 mL of saline.
The CEUS was performed to study the healing of the lesions, and conventional ultrasound was performed to detect free intraperitoneal fluid at d 3, 7, and 10 postoperatively. To avoid bias in the measurements, all animals were placed in a supine position. A bolus of SonoVue™ (0.025 mL/kg) was administered through an accessory cephalic vein, followed by a 5 mL normal saline flush. The CEUS revealed non- and/or hypo-enhanced regions in the liver injury sites after the focal injection (Figure 1). For the off-line analysis, digital images were recorded on the scanner as single-frame pictures and multiple cine loops.
Hemostatic clamp uniformly positioned over the left lateral lobe of the liver to inflict a 4 cm×4 cm×2 cm blunt liver injury (AAST grade III–IV). The application of the injection was begun at the deepest point of the liver lesion directly with a standard syringe. After injection SonoVue, hepatic blunt trauma was shown as a non and hypo-enhanced region (arrows) on CEUS image.
Statistical analysis
The values were depicted as the mean±standard deviation (SD) with P<0.05 defined as significant. Differences between the time to achieve hemostasis and the weight of blood loss in the different groups were determined using the t test. Measurements taken in each dog (ascites and lesion area) were analyzed using one-way ANOVA and multiple comparisons according to Dunnett T3. Measurements taken in each dog (hematology and biochemistry parameters) were analyzed using one-way ANOVA and multiple comparisons according to Bonferroni. The data were analyzed using the SPSS17.0 statistical package.
Results
There was no difference in the body weight of the animals between the two groups (the body weights in the treatment and control groups were 16.82±1.06 kg and 16.73±1.15 kg, respectively (P>0.05). All the dogs survived after the procedure due to complete hemostasis.
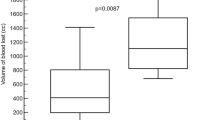
The mean time to achieve complete hemostasis in the treatment group (range 1.00–1.83 min) after the application of the novel hemostatic sealant without additional compression was shorter than that in the thrombin group (range 5.83–7.83 min). There was a significant decrease in the average blood loss in the treatment group (range 37.91–52.52 mL) compared with that in the thrombin group (range 64.82–105.72 mL) (Table 1).
Conventional ultrasound was performed during the 10 d follow-up to detect the presence of free intraperitoneal fluid (Figure 2). In the treatment group, ascites were not found in any animal (n=10) at d 3, 7, and 10 postoperatively, whereas in the control group, ascites were found in all animals (n=10) at d 3, in 2 dogs (2/10) at d 7 and in 0 dogs at d 10. The largest diameter of the ascites in the control group was 0.59±0.25 cm at d 3 and 0.06±0.13 cm at d 7.
Comparison of the free intraperitoneal fluid between the two groups at different time points. bP<0.05 indicates differences between the treatment and control groups at the same time interval.
The lesion sites were measured by CEUS during the 10 d follow-up (Figure 3). In the treatment group, hepatic lesion were found in all animals (n=10) at d 3 postoperatively, but no lesions were found at d 7 and 10 postoperatively. In the control group, hepatic lesions were found in all animals (n=10) at d 3 and 7 postoperatively, and lesions still existed in 2 dogs at d 10 postoperatively. At d 3, the difference in the area of the hepatic lesions was significant between the 2 groups (1.65±0.72 cm2 vs 2.62±0.49 cm2, P<0.05). The area of the lesions in the control group was 1.47±0.62 cm2 at d 7 and 0.23±0.51 cm2 at d 10.
Comparison of the areas of the hepatic lesions between the treatment and control groups at different time points. bP<0.05 indicates differences between the two groups at the same time interval.
The hematology parameters at three different time points were also compared between the two groups (Table 2). The mean values of hematocrit and hemoglobin both increased gradually over time. Although the mean values for hematocrit and hemoglobin were not within the normal range for dogs in the control group at d 3 postoperatively, there were no significant differences from the treatment group at the same time point (P>0.05 for both). The mean values for the biochemistry parameters at three different time points were also compared between the two groups (Table 3). The mean values of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) both decreased gradually in the procedure. There were no significant differences between the two groups (P>0.05 for all). At d 10 postoperatively, ALT and AST in both groups returned to the normal range.
Discussion
Patients and surgeons have recognized that underlying benefits might be produced by the timely control of bleeding, considering the morbidity, mortality and extended hospitalization due to uncontrolled severe traumatic or surgical bleeding30. Bleeding insofar as mild, moderate, or even severe intensity can be quickly and successfully treated with traditional mechanical methods (ie, pressure, suturing, ligature, clips and staples) or with various electrosurgical devices in most cases31. Because of their inadequate surgical technique, topical hemostats are typically utilized when traditional measures are ineffective or impractical.
So far, a wide variety of topical hemostats have been developed and are available to assist surgeons in the perioperative management of bleeding, including mechanical agents (such as gelatin foams, oxidized regenerated celluloseand microfibrillar collagen) and active hemostats (such as topical thrombin)32. However, the performance of current hemostatic materials is far from ideal. The efficacy of mechanical agents requires an intact coagulation cascade to ensure that fibrin is produced, and thus these agents will not be effective when hemorrhaging is caused by a significant coagulopathy33. In addition, the acidic nature of oxidized regenerated cellulose may also increase the chance of the inflammation of surrounding tissue and delay wound healing34. More importantly, their uninjectable property considerably restricts their application in interventional treatments. Thrombin has been reported to be purified from numerous sources and has been used as a clinical aid for topical hemostasis for more than 60 years. Thrombin alone may have some effects on diffusely bleeding surfaces, but the clot formed is relatively soft and unstable as thrombin does not provide a framework to which the clot can adhere.
Biomaterials with the capacity to form gels in situ have been attracting considerable attention for a variety of soft tissue applications. In situ gel-forming materials are a class of materials that can undergo transition from a liquid state to a gel at the application site. These hydrogels can attach to a tissue surface to form a physical barrier or seal, and they have been exploited for broad applications, including hemostasis, wound healing, suture support, tissue sealing and drug delivery35,36,37. Interest in soft tissue adhesives is growing because of the desire to replace or supplement sutures for wound closure38, the trends toward less invasive and cosmetic surgeries, and the need for emergency hemostasis39.
Different strategies have been used to obtain in situ-forming hydrogels. One approach commonly used for in vitro gel formation is to initiate polymerization reactions in the presence of multi-functional monomers. Transglutaminase catalyzes the crosslinking reaction between glutamine and lysine residues of proteins. Hydrogels catalyzed by this enzyme formed more slowly and were stronger and permanent40. Previous in vitro studies have shown that the TG adhesive can bond with moist or wet tissue and that the adhesive strength is comparable with, or better than, fibrin-based sealants41,42,43.
CEUS is based on the ability of microbubbles containing gases to produce real-time, contrast-related, gray-scale images. It can clearly show the site and extent of lesions, whether the organ capsule was involved and whether active hemorrhaging still exists. A needle can also be precisely inserted into hepatic lesions under sonographic guidance percutaneously. In addition, it possess more advantages than other minimally invasive methods, considering its advanced properties and functions such as light, fast and wide accessibility, bedside applicability and shielding patients from the hazard of ionizing radiation. The literature44,45,46 indicates that CEUS was effective in the diagnosis of blunt hepatic trauma, had similar performance to that of CT and was more sensitive than conventional sonography.
To the best of our knowledge, this study is the first to investigate the homeostatic efficacy of a novel sealant based on the in situ gel formation of gelatin catalyzed by thrombin and crosslinked by TG for hepatic trauma in a canine model. Significantly lower blood loss and shorter hemostasis times were observed in the treatment group compared with the control group (P<0.05). No ascites were found during the 10 d follow-up, and no hepatic lesions were observed by CEUS on d 7 and 10 postoperatively in the treatment group. In the control group, however, ascites still existed on d 3 and 7 postoperatively, and hepatic lesions were still present on d 10 postoperatively. One explanation for these results may be that gelatin crosslinked by TG can form a gel that offers substantial adhesive strength, enabling the thrombin solution to run off the wound surface and maintain its solid or pasty consistency when combined with a gelatin to facilitate the formation of a stable clot at the bleeding site.
Potential limitations of this study should be acknowledged. First, the left lateral lobe of the liver was chosen as a model, which was reported in a previous study7, and the bleeding here is relatively slow. Thus, further studies are necessary to determine the efficacy of the novel hemostatic sealant in situations with more bleeding. Second, the gelatin solution can form a physical gel at room temperature. Such a physical gel would need to be melted by warming up to 37 °C prior to use, which would be inconvenient for surgeons.
In conclusion, a hemostatic sealant based on in situ gel formation catalyzed by thrombin and crosslinked by gelatin may function in an effective way in hemostasis and promote wound healing after hepatic trauma without additional compression, which eliminated the safety concerns associated with glutaraldehyde. The hemostatic efficacy of the novel hemostatic sealant is better than that of thrombin.
Author contribution
Xia XIE and Jiang-ke TIAN designed the research, collected the data and conducted part of the operations; Jie TANG and Ya-qin HUANG planned and coordinated the research; Rong WU and Wen-bo TANG collected the data, conducted part of the operations and analyzed the data; Fa-qin LV and Yu-kun LUO planned and oversaw the research project and drafted the paper.
References
Hoyt DB, Bulger EM, Knudson MM, Morris J, Lerardi R, Sugerman HJ, et al. Death in the operating room: an analysis of a multi-center experience. J Trauma 1994; 37: 426–32.
Chapman WC, Singla N, Genyk Y, McNeil JW, Renkens KL, Revnolds TC, et al. A phase 3, randomized, double-blind comparative study of the efficacy and safety of topical recombinant human thrombin and bovine thrombin in surgical hemostasis. J Am Coll Surg 2007; 205: 256–65.
Spotnitz WD, Burks S . Hemostats, sealants, and adhesives: components of the surgical toolbox. Transfusion 2008; 48:1502–16.
Nasso G, Piancone F, Bonifazi R, Romano V, Visicchio G, De Filippo CM, et al. Prospective, randomized clinical trial of the FloSeal matrix sealant in cardiac surgery. Ann Thorac Surg 2009; 88: 1520–6.
Cravello L, Mimari R, Agostini A, Pelleqrin V, Limet L, Bartoli JM . Uterine artery embolisation to treat severe haemorrhage following legal abortion. J Gynecol Obstet Biol Reprod (Paris) 2007; 36: 500–2.
Renkens KL Jr, Payner TD, Leipzig TJ, Feuer H, Morone MA, Koers JM, et al. A multicenter, prospective, randomized trial evaluating a new hemostatic agent for spinal surgery. Spine (Phila Pa 1976) 2001; 26: 1645–50.
Leixnering M, Reichetseder J, Schultz A, Fiql M, Wassermann E, Thurnher M, et al. Gelatin thrombin granules for hemostasis in a severe traumatic liver and spleen rupture model in swine. J Trauma 2008; 64: 456–61.
Parviainen V, Joenväärä S, Tukiainen E, Llmakunnas M, Isoniemi H, Renkonen R . Relative quantification of several plasma proteins during liver transplantation surgery. J Biomed Biotechnol 2011; 2011: 248613.
Tagaya N, Abe A, Kubota K . Needlescopic surgery for liver, gallbladder and spleen diseases. J Hepatobiliary Pancreat Sci 2011; 18: 516–24.
Hick EJ, Morey AF, Harris RA, Morris MS . Gelatin matrix treatment of complex renal injuries in a porcine model. J Urol 2005; 173: 1801–4.
Gill IS, Ramani AP, Spaliviero M, Xu M, Finelli A, Kaouk JH, et al. Improved hemostasis during laparoscopic partial nephrectomy using gelatin matrix thrombin sealant. Urology 2005; 65: 463–6.
Desai PJ, Maynes LJ, Zuppan C, Berqe, KA, Torrey R, et al. Hand-assisted laparoscopic partial nephrectomy in the porcine model using gelatin matrix hemostatic sealant without hilar occlusion. J Endourol 2005; 19: 566–9.
Baumann A, Caversaccio M . Hemostasis in endoscopic sinus surgery using a specific gelatin-thrombin based agent (FloSeal). Rhinology 2003; 41: 244–9.
Angioli R, Muzii L, Montera R, Damiani P, Bellati F, Plotti F, et al. Feasibility of the use of novel matrix hemostatic sealant (FloSeal) to achieve hemostasis during laparoscopic excision of endometrioma. J Minim Invasive Gynecol 2009; 16: 153–6.
Madhuri TK, Tailor A, Butler-Manuel S . Use of surgical sealant in debulking surgery for advanced ovarian carcinoma-case report. Eur J Gynaecol Oncol 2010; 31: 582–3.
Chen T, Embree HD, Brown EM, Taylor MM, Payne GF . Enzyme-catalyzed gel formation of gelatin and chitosan: potential for in situ applications. Biomaterials 2003; 24: 2831–41.
Ballantyne B, Jordan SL . Toxicological, medical, and industrial hygiene aspects of glutaraldehyde with particular reference to its biocidal use in cold sterilization procedures. J Appl Toxicol 2001; 21: 131–51.
Babin H, Dickinson E . Influence of transglutaminase treatment on the thermoreversible gelation of gelatin. Food Hydrocoll 2001; 15: 271–6.
Motoki M, Seguro, K . Transglutaminase and its use for food processing trends. Food Sci Technol 1998; 9: 204–10.
Yokoyama K, Nio N, Kikuchi Y . Properties and applications of microbial transglutaminase. Appl Microbiol Biotechnol 2004; 64: 447–54.
Jurgensen K, Aeschlimann D, Cavin V, Genge M, Hunziker EB . A new biological glue for cartilage-cartilage interfaces: tissue transglutaminase. J Bone Joint Surg Am 1997; 79: 185–93.
Tan Y, Chen X, Huang JH, Huang DP, Wang JH . Eficacy of microbial transglutaminase on wound healing in rats. Chin Biotech 2007; 27: 85–90.
Karpuj MV, Becher NW, Springer JE, Chabas D, Youssef S, Pedotti R, et al. Prolonged survival and decreased abnormal movements in transgenic model of Huntington disease, with administration of the transglutaminase inhibitor cystamine. Nat Med 2002; 8: 143–9.
Fuchsbauer HL, Gerber U, Engelmann J, Seeger T, Sinks C, Hecht T . Influence of gelatin matrices cross-linked with transglutaminase on the properties of an enclosed bioactive material using beta-galactosidase as model system. Biomaterials 1996; 17: 1481–8.
Lim LT, Mine Y, Tung MA . Barrier and tensile properties of transglutaminase cross-linked gelatin films as affected by relative humidity, temperature, and glycerol content. J Food Sci 1999; 64: 616–22.
Bohidar HB, Maity S . Polarized light scattering study from gelatin solutions and gels. Eur Polym J 1998; 34: 1361–70.
Liu Y, Kopelman D, Wu LQ, Hijji K, Attar I, Preiss-Bloom O, et al. Biomimetic sealant based on gelatin and microbial transglutaminase: an initial in vivo investigation. J Biomed Mater Res B Appl Biomater 2009; 91: 5–16.
Moore EE, Cogbill TH, Jurkovich GJ, Shackford SR, Malangoni MA, Champion HR . Organ injury scaling: spleen and liver (1994 revision). J Trauma 1995; 38: 323–4.
Morel DR, Schwieger I, Hohn L, Terrettaz J, Llull JB, Cornioley YA, et al. Human pharmacokinetics and safety evaluation of SonoVue, a new contrast agent for ultrasound imaging. Invest Radiol 2000; 35: 80–5.
Hutchinson RW, Broughton D, Barbolt TA, Prandl T, Muench T, Rockar R, et al. Hemostatic effectiveness of Fibrin pad after partial nephrectomy in swine. J Surg Res 2011; 167: 291–8.
Acheson EM, Kheirabadi BS, Deguzman R, Dick EJ Jr, Holcomb JB . Comparison of hemorrhage control agents applied to lethal extremity arterial hemorrhages in swine. J Trauma 2005; 59: 865–74.
Achneck HE, Sileshi B, Jamiolkowski RM, Albala DM, Shapiro MJ, Larson JH . A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann Surg 2010; 251: 217–28.
Sarfati MR, DiLorenzo DJ, Kraiss LW, Galt SW . Severe coagulopathy following intraoperative use of topical thrombin. Ann Vasc Surg 2004; 18: 349–51.
Tomizawa Y . Clinical benefits and risk analysis of topical hemostats: a review. J Artif Organs 2005; 8: 137–42.
Bennett SL, Melanson DA, Torchiana DF, Wiseman DM, Sawhney AS . Next-generation hydrogel films as tissue sealants and adhesion barriers. J Card Surg 2003; 18: 494–9.
Kakinoki S, Taguchi T . Antitumor effect of an injectable in-situ forming drug delivery system composed of a novel tissue adhesive containing doxorubicin hydrochloride. Eur J Pharm Biopharm 2007; 67: 676–81.
Martinez-de-la-Casa JM, Rayward O, Saenz-Frances F, Mendez C, Bueso ES, Garcia-Feijoo J . Use of a fibrin adhesive for conjunctival closure in trabeculectomy. Acta Ophthalmol 2012. DOI: 10.1111/j.1755-3768.2012.02436.x.
Pursifull NF, Morey AF . Tissue glues and nonsuturing techniques. Curr Opin Urol 2007; 17: 396–401.
Pusateri AE, Holcomb JB, Kheirabadi BS, Alam HB, Wade CE, Ryan KL . Making sense of the preclinical literature on advanced hemostatic products. J Trauma 2006; 60: 674–82.
Davis NE, Ding S, Forster RE, Pinkas DM, Barron AE . Modular enzymatically crosslinked protein polymer hydrogels for in situ gelation. Biomaterials 2010; 31: 7288–97.
Chen TH, Embree HD, Brown EM, Taylor MM, Payne GF . Enzyme-catalyzed gel formation of gelatin and chitosan: Potential for in situ applications. Biomaterials 2003; 24: 2831–41.
McDermott MK, Chen TH, Williams CM, Markley KM, Payne GF . Mechanical properties of biomimetic tissue adhesive based on the microbial transglutaminase-catalyzed crosslinking of gelatin. Biomacromolecules 2004; 5: 1270–9.
Chen T, Janjua R, McDermott MK, Bernstein SL, Steidl SM, Payne GF . Gelatin-based biomimetic tissue adhesive. Potential for retinal reattachment. J Biomed Mater Res B Appl Biomater 2006; 77: 416–22.
Catalano O, Lobianco R, Rase MM, Siani A . Blunt hepatic trauma: evaluation with contrast-enhanced sonography: sonographic findings and clinical application. J Ultrasound Med 2005; 24: 299–310.
Yekun L, Shasha W, Xiangsheng Z, Qi C, Guoxin L, Feng H . Contrast-enhanced ultrasound for blunt hepatic trauma: an animal experiment. Am J Emerg Med 2010; 28: 828–33.
Valentino M, Serrra C, Pavilica P, Barozzi L . Contrast-enhanced ultrasound for blunt abdominal trauma. Semin Ultrasound CT MR 2007; 28: 130–40.
Acknowledgements
The financial support of the National Natural Science Foundation of China (No 81071279) is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, X., Tian, Jk., Lv, Fq. et al. A novel hemostatic sealant composed of gelatin, transglutaminase and thrombin effectively controls liver trauma-induced bleeding in dogs. Acta Pharmacol Sin 34, 983–988 (2013). https://doi.org/10.1038/aps.2013.17
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2013.17
Keywords
This article is cited by
-
Plasma-based fast-gelling biohybrid gels for biomedical applications
Scientific Reports (2019)