Abstract
Efficient endocytosis is crucial for maintaining synaptic transmission because of its role in retrieving constituent membrane and associated proteins. In the past three decades three modes of endocytosis have been proposed involving the central nervous system: clathrin-mediated endocytosis, kiss-and-run endocytosis and bulk endocytosis. These forms of endocytosis can be induced under different conditions, but their detailed molecular mechanisms and functions are largely unknown. Here, we review the existence and initiation of all three modes of endocytosis at a giant glutamatergic synapse, the calyx of Held. The possibility of direct electrophysiology recording in this synapse allows for accurate tracking of exocytosis and endocytosis via capacitance measurements. Future aims will be focused on identifying the molecules that undergo the different mechanisms of endocytosis and the conditions under which different forms of endocytosis predominate.
Similar content being viewed by others
Introduction
Neuronal communication relies on synaptic transmission. In the central nervous system (CNS), neurons can fire in the range of 10–100 Hz. However, typical presynaptic terminals in the CNS only have several hundred synaptic vesicles per active zone (AZ). Therefore, efficient endocytosis is crucial to prevent complete exhaustion of secretion-competent vesicles and also to help maintain normal terminal morphology by retrieving excess membrane. A good example of the importance of endocytosis is shibire, a temperature-sensitive phenotype in Drosophila. In this model, neurons cannot retrieve synaptic vesicles at high temperatures, eventually leading to paralysis and falling.
Although the exact mechanisms by which the presynaptic terminal retrieves membrane upon exocytosis are still unclear, there are three hypothetical forms of endocytosis (Figure 1): clathrin-mediated endocytosis (CME), a fast recapture of vesicles called kiss-and-run endocytosis, and bulk endocytosis1, 2, 3, 4. In 1973, Heuser and Reese published their pioneer electron microscopy (EM) findings describing the CME model in the frog neuromuscular junction (NMJ)2 . In this model, the vesicles fully collapse with the plasma membrane and are retrieved by CME. The coated pit and cisternae are frequently observed under 10 Hz stimulation for 1 min. In the same year, Ceccarelli demonstrated a second model of endocytosis. Following 2 h of low-frequency stimulation at 2 Hz, the synaptic vesicle was released and retrieved at the same site instead of forming clathrin-coated vesicles3. This mode was later named “kiss-and-run” endocytosis5. Bulk endocytosis, which is a phenomenon in which a large piece of membrane is retrieved from the presynaptic plasma membrane, has also been frequently observed in several model cells, including the frog NMJ4, rat hippocampal neurons6 and goldfish bipolar cells7. Here, we review the evidence, the mechanisms, and the physiological importance of all three modes of endocytosis at the calyx of Held, a giant synapse mediating auditory processing in the brain stem8.
Multiple modes of endocytosis at the frog NMJ. Three modes of synaptic vesicle retrieval from the frog NMJ. Top: Left, clathrin-mediated endocytosis demonstrated by Heuser and Reese. Right, EM figure shows coated pits and vesicles (arrowed) and cisternae (c). Middle: Left, synaptic vesicles are retrieved at the same site, later named “kiss-and-run”. Right, EM figure shows no clathrin-coated vesicles and cisternae at low stimulation frequency. Bottom: Left, bulk endocytosis, in which a large piece of membrane internalises after full fusion. New coated vesicles may bud from it or from large, internalised cisternae. Right, EM figure shows internalised cisternae (open arrows: empty; filled arrows: filled with FM1-43). Scale bar: 250 nm in all EM figures. Reprinted by permission from John Wiley and Sons Ltd: Journal of Physiology1, copyright 2003.
Capacitance measurement at the calyx of Held
Although clathrin-dependent endocytosis is well accepted, the existence of kiss-and-run and bulk endocytosis and their physiological importance are still debated owing to a lack of direct evidence more than three decades after their initial description (See Table 1 for the debate on kiss-and-run). One major difficulty is resolving the rapid fusion and fission processes.
Capacitance measurement, a powerful technique with a time resolution of approximately 1 ms, has been applied to address these problems. The principle on which this technique is based is quite simple: the capacitance of the membrane is proportional to its surface area9. When synaptic vesicles fuse with the plasma membrane, the surface area of the presynaptic membrane increases, thereby increasing membrane capacitance. Therefore, an increase in capacitance indicates exocytosis, whereas a decrease indicates endocytosis. The major drawback of this technique is that it requires patch clamp recordings at large nerve terminals. Certain invertebrate giant synapses have been used to study presynaptic functions10, 11. However, similar studies in vertebrate synapses are more difficult due to the technical complexities involved in patch clamp recording from small nerve terminals.
The calyx of Held is a very large glutamatergic synapse of the auditory pathway in the brain stem and is suitable for studying fast transmitter release in the vertebrate CNS (Figure 2A)12. The calyx of Held originates from globular bushy cells (GBC) in the anterior ventral cochlear nucleus (aVCN) and synapses onto a principal cell in the medial nucleus of the trapezoid body (MNTB). Direct electrophysiological recordings can be made simultaneously from both the nerve terminal and the principal cell (Figure 2B). These properties confer unique advantages in the study of the presynaptic mechanisms of transmitter release and short-term plasticity. This model also allows us to characterize the different kinetics involved in endocytosis13, 14, 15, 16.
Diagram of the calyx of Held in the mammalian auditory pathway. (A) The calyx of Held functions in sound localization and is located between globular bushy cells (GBCs) and the principal cells of the contralateral medial nucleus of the trapezoid body (MNTB). The calyx of Held is axosomatic and glutamatergic, whereas the principal cell of the MNTB is glycinergic. Reprinted by permission from Macmillan Publishers Ltd: Nature Neuroscience8, copyright 2002. (B) Left: an infrared differential interference contrast (IR-DIC) image of a calyx of Held and principal cell in a brain slice. Right: pseudo-color image of a calyx and principal cell. The presynaptic terminal was filled with Lucifer Yellow and the postsynaptic cell with Cascade Blue. The two pipettes demonstrate the possibility of simultaneous recordings from both pre- and postsynaptic cells. Reproduced by permission from John Wiley and Sons Ltd: Journal of Physiology12, copyright 1995.
Rate of endocytosis depends on stimulation intensity
Several studies have determined that the rate of endocytosis depends on stimulation intensity13, 17, 18. A mild stimulation (action potential equivalent, AP-e) results in only brief endocytosis. A 50 AP-e stimulation at 100 Hz causes an abrupt increase of capacitance, indicating exocytosis. This is followed by a slow decrease in capacitance, indicating endocytosis with a time constant of 10–20 s. If the stimulation intensity is further increased to 200 AP-e at 100 Hz, the increase in capacitance is higher, and a rapid endocytosis with a time constant of 1–2 s occurs, followed by slow endocytosis (Figure 3A). Rapid endocytosis may be physiologically important during high frequency stimulation because of increased intracellular calcium18.
The rate of endocytosis depends on stimulation intensity. (A) Sampled capacitance traces induced by single AP-e (1 ms depolarization from -80 to +7 mV, left), 50 AP-e (middle) and 200 AP-e (right). A fast endocytosis followed by slow endocytosis was observed using 200 AP-e. Scale bars are different in each of the stimulation conditions. Reproduced from Wu et al (2005)18, copyright by the Journal of Neuroscience. (B) Left: sampled membrance capacitance (Cm) traces induced by ten depolarizing pulses of 20 ms from -80 to +10 mV at 10 Hz. Arrow indicates a DCS (downward capacitance shift). Right: plot of the DCS using a larger scale. Note that the DCS is about 100 fF, equivalent to more than 1000 regular vesicles. Reproduced from Wu et al (2007)20, copyright by the Proceedings of the National Academy of Sciences.
Under certain strong stimulation protocols (for example, 10 pulses of 20 ms stimulation at 10 Hz), bulk endocytosis is observed, which is characterized by a large downward shift in capacitance of approximately 50–300 ms (Figure 3B). For a single vesicle, the average membrane capacitance is 23–220 attofarads (aF) with a mean of 73 aF19. This decrease in capacitance under strong stimulation therefore reflects the endocytosis of a very large piece of membrane. The decrease in capacitance in these cases may be on the order of 100 femtofarads (fF), equivalent to a simultaneous retrieval of 1000–2000 synaptic vesicles19, 20.
Cell-attached recordings characterize all three modes of endocytosis
The rapid and slow types of endocytosis observed in the calyx of Held may reflect the action of kiss-and-run endocytosis and CME, respectively. Studies from bipolar cells, however, have shown that full collapse fusion could also be followed by rapid endocytosis21.
In addition to the rate of endocytosis, changes in fusion pore conductance are also used as criteria when distinguishing between kiss-and-run and the full collapse model. A rapid opening and closing of the fusion pore indicates kiss-and-run, whereas fusion pore opening followed by expansion indicates full collapse fusion. Addressing this question requires a similar but different category technique, called cell-attached recording. The signal-to-noise ratio is improved with this technique, and only responses from the small piece of membrane clamped to the electrode are recorded.
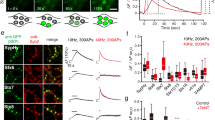
Ling-gang WU's group successfully made the first cell-attached recordings at the release sites of the calyx of Held by removing the principal neuron with a large pipette19 (Figure 4A). When the nerve terminal was depolarized through the addition of a high concentration of KCl in order to trigger vesicle fusion, the magnitude of the small up-steps in the capacitance, indicating exocytosis, agreed with the single-vesicle capacitance estimated from EM studies22 (Figure 4B). Approximately 20% of the time, capacitance flicker was observed. The term “flicker” is used because the capacitance trace displayed up- and down-steps of similar size with durations of 10 ms to 2 s, suggesting that each step was due to the movement of the same vesicle (Figure 4B). In some cases, fusion pore conductance could be detected with a mean conductance of about 66 picosiemens (ps), equivalent to a fusion pore diameter of about 1 nm (Figure 4C). Within 200 ms, the fusion pore opens, pauses and closes, indicating kiss-and-run endocytosis. This capacitance flicker provides a best piece of evidence for the existence of kiss-and-run endocytosis at CNS synapses. The rapid opening and closing of the fusion pore involved in kiss-and-run endocytosis can control quantal release and thus influence post-synaptic response19, 23.
Multiple modes of endocytosis using cell-attached recording at the calyx of Held. (A) The procedure of cell-attached recording at the release site of the calyx. A large pipette was used to remove the postsynaptic principal cell, and a second, smaller pipette was used to make recordings at the release site. The green pseudo-color shows the presynaptic terminal filled with Lucifer Yellow. (B) Top: sampled capacitance trace induced by high KCl stimulation (50–100 mmol/L). Inset shows trace using a larger scale. Im (imaginary part of admittance) reflects capacitance. Up-steps indicate exocytosis upon stimulation. Bottom: a second sample trace shows capacitance flicker and full collapse fusion. Note that the up- and down-step of the flicker has a similar size (about 50 aF), suggestive of a single-vesicle event. (C) Sample Im, Re (real part of admittance) and Gp (conductance of fusion pore) traces of a full collapse event. Note that only a small fraction of the Gp can be detected because of the rapid expansion of fusion pore. A–C are reprinted by permission from Macmillan Publishers Ltd: Nature19, copyright 2006.
In the majority of the fusion events, the up-steps of exocytosis are not followed until much later by the down-steps of endocytosis, presumably reflecting slow endocytosis. In cases where the fusion pore conductance can be measured, the fusion pore opens and expands to an undetectable level within 10–300 ms, demonstrating the full collapse of vesicle fusion19.
Furthermore, about 20% of the capacitance down-step events induced by stimulation (high KCl or current injection) were large down-steps, with a mean of about 600 aF (ranging from 220–3000 aF). This is a much larger shift than could be explained by a conventional single vesicle's capacitance (22–220 aF, with a mean of about 73 aF). These large down-steps probably reflect bulk endocytosis, which we discuss in a later section24.
In summary, during the course of cell-attached recordings under high KCl stimulation, the majority of fusion events suggest full collapse with slow endocytosis. Only a small percentage indicate either kiss-and-run endocytosis with different fusion pore sizes or rapid bulk endocytosis.
Calcium/calmodulin triggers all forms of endocytosis
How the different forms of endocytosis are initiated has remained an incompletely resolved question since the discovery of endocytosis three decades ago. Our lack of understanding is likely due to the difficulty in distinguishing between exocytosis and endocytosis. In a recent study25, when 10 mmol/L of BAPTA (a fast calcium buffer) was added to the presynaptic pipette solution, only a small amount of exocytosis was observed, indicating that the buffer blocked a large amount of calcium-dependent exocytosis. Endocytosis, however, was completely blocked (Figure 5A). This simple experiment indicates that endocytosis is calcium-dependent25. Given that calcium is the first signal to trigger exocytosis, it may also be an initiator of endocytosis. If this hypothesis is correct, endocytosis will be slowed or completely inhibited in a low-calcium environment. Conversely, a calcium-initiated model of endocytosis would predict accelerated endocytosis in a high-calcium environment. The inhibition of endocytosis was tested using double-patch recordings at the calyx of Held nerve terminals and principal neurons using a presynaptic pipette solution containing 750 nmol/L calcium. Presynaptic membrane capacitance was measured and analysed in conjunction with postsynaptic miniature excitatory postsynaptic current (mEPSC) events. mEPSC responses reflect only the exocytosis of the presynaptic membrane, whereas presynaptic membrane capacitance demonstrates the net effect between exocytosis and endocytosis. When capacitance increase data were converted to vesicle releases (about 65–73 aF/vesicle), these data closely matched the mEPSC events (a single mEPSC event reflects a single vesicle fusion event). This nearly perfect match indicates the extreme reduction of endocytosis in this condition25.
Calcium influx triggers all forms of endocytosis. (A) Endocytosis is initiated by calcium. Left: Cm increase is induced by ten pulses of 20 ms stimulation, depolarized from -80 to +10 mV at 10 Hz. Right: trace from the same stimulation protocol with 10 mmol/L BAPTA in the pipette solution. Most of the exocytosis events were blocked, whereas a small fraction remained. Endocytosis, however, was fully blocked. The scale bars differ between the two conditions. (B) Increasing calcium influx accelerates endocytosis. A bath solution containing 5.5 mmol/L calcium and a stimulation protocol of ten pulses of 50 ms at 10 Hz induced an obvious overshoot. (C) Calcium influx triggers bulk endocytosis and accelerates fission pore closure. Left: ten pulses of 50 ms at 10 Hz induced rapid bulk endocytosis. Right: with 70 mmol/L EGTA, the same stimulation protocol induced fewer and slower bulk endocytosis events. A larger scale of the DCS and a calculation of the Gp are plotted to the right. A–C are reproduced from Wu et al (2009)25.
The speeding up of endocytosis was also tested by increasing the stimulation intensity. Ten stimulating pulses at 10 Hz were applied to the nerve terminal with different pulse durations (ranging from 2 to 50 ms) to increase calcium influx. Endocytosis was seen to accelerate following this protocol. If the calcium concentration of the bath solution was increased from 2 mmol/L to 5.5 mmol/L and a 50-ms pulse train was used, both fast endocytosis and an overshoot of endocytosis were observed (Figure 5B). Endocytosis overshoot indicates that the amount of endocytosis is more than the immediately preceding exocytosis25.
Calcium has also been shown to initiate bulk endocytosis. The addition of 70 mmol/L of EGTA (a slow calcium buffer) to the presynaptic pipette solution dramatically decreased the probability of detecting bulk endocytosis. Furthermore, fusion pore closure was seen to be much slower in the presence of EGTA25, demonstrating that calcium also controls the fission rate of bulk endocytosis (Figure 5C).
Calmodulin (CaM) is a calcium sensor that mediates all forms of calcium-dependent endocytosis. Using different kinds of calmodulin blockers differentially inhibits either slow or fast endocytosis. Bulk endocytosis can also be blocked by the calmodulin blocker calmidazolium25.
Compound vesicle fusion and bulk endocytosis
It is known that there are three forms of endocytosis: clathrin-mediated slow endocytosis, kiss-and-run endocytosis, and bulk endocytosis. It has also been established that there are two forms of vesicle fusion: kiss-and-run fusion and full collapse fusion. Bulk endocytosis does not appear to correlate with any known form of exocytosis. In 2009, a new mode of exocytosis, called compound exocytosis, was proposed in the calyx of Held24. Compound fusion describes a kind of fusion among small vesicles forming large vesicles. These large vesicles have an increased quantal size and undergo compound exocytosis followed by bulk endocytosis.
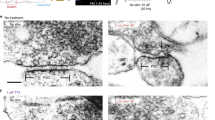
Up-steps and down-steps in capacitance detected by cell-attached recordings at release sites of the calyx of Held reflect exocytosis and endocytosis, respectively. In control conditions, most of the up- and down-steps are smaller than about 220 aF, which is a regular vesicle's maximal capacitance, based on findings from EM studies22. This suggests that most of the observed releases are from single vesicles. However, when high KCl (about 75 mmol/L) was applied to stimulate the cell, about 20% of the up-steps were larger than 220 aF with an average of about 600 aF (Figure 6A, 6B). This suggests the possibility of compound fusion among regular vesicles. EM studies have also confirmed this phenomenon. Large vesicle-like structures were observed after 50–100 mmol/L KCl stimulation for about 2 min, which could be interpreted as compound fusion26(Figure 6C). Recordings of mEPSCs from postsynaptic cells in the presence of high KCl also revealed extraordinarily large mEPSC responses (100–300 pA), further implying that compound fusion could increase quantal size27, 28, 29, 30 (Figure 6D, top). Together, these three pieces of evidence suggest the existence of compound exocytosis.
Calcium/synaptotagmin-II-mediated compound vesicle fusion followed by bulk endocytosis. (A) Large up- and down-steps in capacitance can be detected under high KCl stimulation. Left: a sample Im trace shows large up-steps, which reflect compound exocytosis. Right: two sample Im traces show large down-steps, which may reflect bulk endocytosis. (B) Synaptotagmin-II mediates compound vesicle fusion. Left: in wild-type mice, similar large up-steps were observed as were seen in rats. Right: in syt2−/− knockout mice, only small up-steps (<220 aF) were observed. (C) EM images of the calyx in rats and syt2−/− knockout mice. In rats, large vesicle-like structures were observed after about 2 min of high KCl stimulation. In syt2−/− knockout mice, only small vesicles were observed after the same stimulation. (D) Compound vesicle fusion triggers large mEPSCs at the postsynaptic neuron. Top: in wild-type mice, large mEPSCs (100–300 pA) could be induced after high KCl stimulation. Bottom: in syt2−/− knockout mice, compound fusion was blocked; therefore, no large mEPSC was detected. (E) PTP and quantal size increase are calcium/synaptotagmin-II dependent. Left: in wild-type mice, fiber stimulation of 30 s at 100 Hz induced a fast component of PTP followed by a slow component. The fast component is at least partially PKC-dependent. The slow component is dominated by the quantal size increase and is PKC-independent. (F) The new model for vesicle exocytosis and endocytosis at synapses. Endo: endocytosis; kiss-and-run: kiss-and-run fusion and retrieval; exo: exocytosis. A-F are reproduced with modifications from He et al (2009)24.
Compound fusion is observed in both rats and mice. However, in synaptotagmin-II knockout (syt2−/−) mice, high KCl failed to induce large up- or down-steps in capacitance; only regular up- and down-steps were observed (Figure 6B, right). Moreover, large vesicle-like structures were not visible by EM following stimulation with high KCl (Figure 6C). Furthermore, the mEPSC measured from the postsynaptic cells showed no large events, indicating a small quantal size (Figure 6D, bottom). Synaptotagmin is an immediate calcium sensor that mediates calcium-dependent exocytosis, and in the calyx of Held, synaptotagmin-II mediates this rapid fusion event31. These results together suggest that, similarly to regular fusion events, compound fusion requires synaptotagmin. Experiments have also shown that compound fusion requires calcium. Neither regular nor large up- or down-steps in capacitance are observed when neurons are stimulated with high KCl in a calcium-free extracellular solution.
Calcium and synaptotagmin-II mediate compound fusion24. However, all results showing this effect were obtained under high KCl, which is non-physiological condition, raising the question of whether compound fusion also occurs under physiological conditions. Since calyx of Held synapses can fire at rates of up to several hundred Hz of stimulation for relatively long periods of time, Ling-gang WU's group applied 100 Hz stimulation for 20 s to mimic physiological stimulation that may occur in vivo during auditory processing. Capacitance recordings from release sites showed that during, and even after, stimulation, large up-steps larger than 220 aF were observed, further confirming the existence of compound fusion24.
One prediction regarding compound fusion is that it would increase quantal size and synaptic strength. Large mEPSC (100–300 pA) have been demonstrated under high KCl stimulation (Figure 6D, top), suggesting an increase in quantal size. To test the effect of compound fusion on synaptic strength, the same research group applied axonal stimulation to generate action potentials in the nerve terminals and measured mEPSCs along with evoked-EPSCs from the post-synaptic cells24. When a 100-Hz train of stimulation was applied, the evoked-EPSC increased immediately, a well-known phenomenon known as PTP (post-tetanic potentiation). PTP was followed by a decay lasting a few minutes32, 33. Interestingly, PTP was accompanied by an increase in mEPSC size. The mEPSC size increase did not match the fast EPSC size increase (PTPfast) immediately following stimulation but overlapped with the EPSC increase after about 1–2 min. A most recent work from this group showed that PTP is composed of two components: a PKC (protein kinase C)-dependent increase of quantal content and a PKC-independent increase of quantal size34. The slow component of PTP (PTPslow) is dominated by the quantal size increase as a result of compound fusion. Because PTP is widely observed at many synapses following intensive stimulation32, 35, in addition to the contributions from postsynaptic effects36, 37, 38, it is proposed that compound fusion, may also play an important presynaptic role in synaptic plasticity35, 39.
Because compound fusion requires calcium and synaptotagmin-II, an intuitive speculation is that the same requirement applies to PTP. PTP in wild-type mice is similar to that observed in rats (Figure 6E, left). In synaptotagmin-II knockout mice, however, EPSC and mEPSC potentiation disappeared following stimulation, which strongly suggests that synaptotagmin-II is required for PTP24, 31 (Figure 6E, right). When EGTA was added to the extracellular solution to buffer calcium, PTP and mEPSC potentiation disappeared, further confirming the requirement of calcium24.
In addition to large up-steps in capacitance, large down-steps were also observed in the same conditions used to observe compound fusion. These down-steps occurred later than the up-steps, were of similar size, and were not observed in synaptotagmin-II knockout mice. These results further suggest the existence of bulk endocytosis followed by compound exocytosis.
Taken into account recently published data, a new recycling model has been proposed24. In this model, compound fusion occurs among small regular vesicles to form large vesicles. These large vesicles have an increased quantal size and undergo compound exocytosis, thereby increasing synaptic strength through PTP. These vesicles are then retrieved by bulk endocytosis (Figure 6F).
Conclusion and outlook
In summary, influxes of calcium into the nerve terminal trigger different forms of exo- and endocytosis. There are three forms of vesicle cycling: 1) kiss-and-run exo-endocytosis, which involves rapid opening and closure of the fusion pore; 2) full collapse of the vesicle membrane with the plasma membrane, followed by a slow clathrin-mediated endocytosis; and 3) compound vesicle fusion followed by bulk endocytosis (Figure 6F). All three forms cooperate to ensure the efficiency of vesicle recycling at the presynaptic nerve terminal. In addition to recycling, these forms of exo- and endocytosis may also regulate quantal size. Kiss-and-run endocytosis utilizes a small fusion pore that can decrease quantal size and may have effects on synaptic strength. Compound fusion can increase quantal size and subsequently induce synaptic potentiation. Each of these mechanisms provides various ways for the terminal to adjust synaptic transmission and plasticity under different physiological conditions. A recent study from Tsien's group elegantly demonstrated the efficiency of vesicle cycling. Using Qdots (quantum dots) to distinguish between the multiple modes of fusion events, they found that different vesicle cycling modes predominated under different conditions. During a train of stimulation, kiss-and-run endocytosis gradually gave way to full collapse endocytosis40.
Although our knowledge of vesicle exocytosis and endocytosis has been greatly expanded in the past decades, many questions remain unanswered. We know that many proteins participate in clathrin dependent endocytosis, although their detailed function and mechanisms are still unclear. Other than the existence of kiss-and-run endocytosis, its physiological function and molecular mechanisms are largely unknown. With respect to bulk endocytosis, the final fission process has not been resolved and its molecular regulation has received little attention. Ongoing studies combining capacitance measurements, scanning ion conductance microscopy (SICM), quantum dots, fluorescent dye imaging and series EM reconstructions may give answers to these questions in the near future.
References
Royle SJ, Lagnado L . Endocytosis at the synaptic terminal. J Physiol 2003; 553: 345–55.
Heuser JE, Reese TS . Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol 1973; 57: 315–44.
Ceccarelli B, Hurlbut WP, Mauro A . Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol 1973; 57: 499–524.
Richards DA, Guatimosim C, Betz WJ . Two endocytic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron 2000; 27: 551–9.
Fesce R, Grohovaz F, Valtorta F, Meldolesi J . Neurotransmitter release: fusion or 'kiss-and-run'? Trends Cell Biol 1994; 4: 1–4.
Takei K, Mundigl O, Daniell L, De Camilli P . The synaptic vesicle cycle: a single vesicle budding step involving clathrin and dynamin. J Cell Biol 1996; 133: 1237–50.
Paillart C, Li J, Matthews G, Sterling P . Endocytosis and vesicle recycling at a ribbon synapse. J Neurosci 2003; 23: 4092–9.
von Gersdorff H, Borst JG . Short-term plasticity at the calyx of Held. Nat Rev Neurosci 2002; 3: 53–64.
Neher E, Marty A . Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci USA 1982; 79: 6712–6.
Augustine GJ, Charlton MP, Smith SJ . Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J Physiol 1985; 367: 163–81.
Young JZ, Keynes R . The Functioning of the Giant Nerve Fibres of the Squid. 1938 - J.Z. and the discovery of squid giant nerve fibres. J Exp Biol 2005; 208: 179–80.
Borst JG, Helmchen F, Sakmann B . Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J Physiol 1995; 489: 825–40.
Sun JY, Wu LG . Fast kinetics of exocytosis revealed by simultaneous measurements of presynaptic capacitance and postsynaptic currents at a central synapse. Neuron 2001; 30: 171–82.
Sun JY, Wu XS, Wu W, Jin SX, Dondzillo A, Wu LG . Capacitance measurements at the calyx of Held in the medial nucleus of the trapezoid body. J Neurosci Methods 2004; 134: 121–31.
Taschenberger H, Leao RM, Rowland KC, Spirou GA, von Gersdorff H . Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron 2002; 36: 1127–43.
Wolfel M, Schneggenburger R . Presynaptic capacitance measurements and Ca2+ uncaging reveal submillisecond exocytosis kinetics and characterize the Ca2+ sensitivity of vesicle pool depletion at a fast CNS synapse. J Neurosci 2003; 23: 7059–68.
Sun JY, Wu XS, Wu LG . Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature 2002; 417: 555–9.
Wu W, Xu J, Wu XS, Wu LG . Activity-dependent acceleration of endocytosis at a central synapse. J Neurosci 2005; 25: 11676–83.
He L, Wu XS, Mohan R, Wu LG . Two modes of fusion pore opening revealed by cell-attached recordings at a synapse. Nature 2006; 444: 102–5.
Wu W, Wu LG . Rapid bulk endocytosis and its kinetics of fission pore closure at a central synapse. Proc Natl Acad Sci USA 2007; 104: 10234–9.
von Gersdorff H, Matthews G . Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature 1994; 367: 735–9.
Satzler K, Sohl LF, Bollmann JH, Borst JG, Frotscher M, Sakmann B, et al. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J Neurosci 2002; 22: 10567–79.
Klyachko VA, Jackson MB . Capacitance steps and fusion pores of small and large-dense-core vesicles in nerve terminals. Nature 2002; 418: 89–92.
He L, Xue L, Xu J, McNeil BD, Bai L, Melicoff E, et al. Compound vesicle fusion increases quantal size and potentiates synaptic transmission. Nature 2009; 459: 93–7.
Wu XS, McNeil BD, Xu J, Fan J, Xue L, Melicoff E, et al. Ca2+ and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat Neurosci 2009; 12: 1003–10.
Matthews G, Sterling P . Evidence that vesicles undergo compound fusion on the synaptic ribbon. J Neurosci 2008; 28: 5403–11.
Heuser JE . Proceedings: A possible origin of the 'giant' spontaneous potentials that occur after prolonged transmitter release at frog neuromuscular junctions. J Physiol 1974; 239: 106P–108P.
Henze DA, McMahon DB, Harris KM, Barrionuevo G . Giant miniature EPSCs at the hippocampal mossy fiber to CA3 pyramidal cell synapse are monoquantal. J Neurophysiol 2002; 87: 15–29.
Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, et al. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci 2000; 3: 1256–65.
Wall MJ, Usowicz MM . Development of the quantal properties of evoked and spontaneous synaptic currents at a brain synapse. Nat Neurosci 1998; 1: 675–82.
Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Sudhof TC . A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature 2007; 450: 676–82.
Korogod N, Lou X, Schneggenburger R . Presynaptic Ca2+ requirements and developmental regulation of posttetanic potentiation at the calyx of Held. J Neurosci 2005; 25: 5127–37.
Habets RL, Borst JG . Post-tetanic potentiation in the rat calyx of Held synapse. J Physiol 2005; 564: 173–87.
Xue L, Wu LG . Post-tetanic potentiation is caused by two signalling mechanisms affecting quantal size and quantal content. J Physiol 2010; 588: 4987–94.
Zucker RS, Regehr WG . Short-term synaptic plasticity. Annu Rev Physiol 2002; 64: 355–405.
Joshi I, Yang YM, Wang LY . Coincident activation of metabotropic glutamate receptors and NMDA receptors (NMDARs) downregulates perisynaptic/extrasynaptic NMDARs and enhances high-fidelity neurotransmission at the developing calyx of Held synapse. J Neurosci 2007; 27: 9989–99.
Joshi I, Wang LY . Developmental profiles of glutamate receptors and synaptic transmission at a single synapse in the mouse auditory brainstem. J Physiol 2002; 540: 861–73.
Malenka RC, Bear MF . LTP and LTD: an embarrassment of riches. Neuron 2004; 44: 5–21.
Oertel D . The role of timing in the brain stem auditory nuclei of vertebrates. Annu Rev Physiol 1999; 61: 497–519.
Zhang Q, Li Y, Tsien RW . The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science 2009; 323: 1448–53.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xue, L., Mei, Ya. Synaptic vesicle recycling at the calyx of Held. Acta Pharmacol Sin 32, 280–287 (2011). https://doi.org/10.1038/aps.2010.212
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.212