Abstract
Junctophilin-2 (JPH2) is a membrane-binding protein that plays a key role in the organization of the junctional membrane complex (JMC) in cardiac myocytes. JPH2 is believed to keep the plasma membrane and sarcoplasmic reticulum at a fixed distance within the JMC, which is essential for proper Ca2+-induced Ca2+ release during the excitation-contraction process. Recent studies have revealed that mutations in the JPH2 gene are associated with hypertrophic cardiomyopathy, highlighting the importance of this protein for normal cardiac physiology. In this paper, we review current knowledge about the structure and function of junctophilin-2 in the heart.
Similar content being viewed by others
Main
Junctophilin-2 (JPH2) is a membrane-binding protein that plays an important role in junctional membrane complexes (JMC) in cardiac myocytes. There are four members in the junctophilin protein family (JPH1-4), and they are believed to bridge the physical gap between the plasma membrane (PM) and the sarcoplasmic/endoplasmic reticulum (SR/ER) in excitable cells. The JPH2 gene encodes the principal junctophilin isoform in the heart, although JPH1 is also expressed to a lesser extent1. JPH1 is the major isoform in skeletal muscle, whereas JPH3 and JPH4 are neuronal isoforms expressed in subsurface cisternae2.
A recent phylogenetic analysis of over 60 JPH genes from over 40 species revealed that junctophilins are highly conserved, in particular the 'membrane occupation and recognition nexus' (MORN) motifs found in the N-terminus of all isoforms3 (Figure 1). In the case of JPH2, eight MORN domains are thought to mediate attachment to the PM, either by binding membrane lipids4 or proteins within the plasma membrane5. The 14-amino acid MORN motifs are highly conserved across isoforms and species, suggesting that these domains are essential for JPH2 function3. Computational models predict the formation of a well-conserved α-helical domain of about ∼100 amino acids, which is believed to provide the structural basis for the distance-spanning feature of the protein1, 3. Each JPH isoform also contains a divergent region, that exhibits a high degree of conservation (83%−91%) across species, but is poorly conserved across the 4 JPH isoforms (15%−17%)3. The function of this domain is presently unknown, although it may play a role in isoform-specific JPH functions. Finally, junctophilins are believed to bind to the ER/SR membrane using a C-terminal, 22-amino acid transmembrane anchor. At present, there is no x-ray crystallography or well-defined structural model available for JPHs.
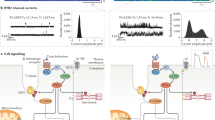
Schematic representation of a junctional membrane complex (JMC) in cardiac myocytes, showing voltage-gated Ca2+ channels (VGCC, also known as L-type Ca2+ channels), cardiac ryanodine receptors (RyR2), and junctophilin-2 (JPH2) proteins. The inset depicts the proposed structural domains of JPH2. PM, plasma membrane; SR, sarcoplasmic reticulum; TMD, transmembrane domain.
Recent studies have begun to uncover the physiological role of JPH2 in cardiac muscle. Germ-line knockout of JPH2 turned out to be lethal in mice1. Although JPH2-/- embryos appeared to develop normally, hearts did not exhibit rhythmic contractility and embryos died by day E10.5. Electron microscopy analysis of ventricular myocytes isolated from E9.5 JPH2−/− embryos revealed a severely decreased number of JMCs1. Moreover, these myocytes exhibited Ca2+ transients with a lower amplitude compared to wild-type controls. Finally, a large number of the JPH2−/− myocytes showed Ca2+ transients that were not evoked by PM depolarization and occurred randomly, suggesting that JPH2 is required for normal SR Ca2+ release1.
Ca2+-induced Ca2+ release from the SR is an essential component of excitation-contraction (EC) coupling and cardiac myocyte function (Figure 2)6. Depolarization of the PM triggers the opening of voltage-gated Ca2+ channels (VGCC), allowing influx of Ca2+ ions into the cytosol. This triggers the release of a greater amount of Ca2+ from the SR via ryanodine receptors (RyR2). After the Ca2+-induced contraction of the sarcomere, myocyte relaxation occurs when Ca2+ ions are pumped back into the SR by sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2a) or Ca2+ is extruded from the myocyte by Na+/Ca2+ exchanger (NCX). Junctophilin is believed to be essential for normal Ca2+-induced Ca2+ release by keeping VGCC and RyR2 Ca2+ channels at a fixed distance within the Ca2+ release unit to ensure stable excitation-contraction coupling1.
Overview of the flow of calcium within the junctional membrane complex during excitation-contraction coupling. Plasma membrane (PM) depolarization triggers the opening of voltage-gated Ca2+ channels (VGCC), allowing influx of calcium ions. This triggers the release of a greater amount of Ca2+ from the sarcoplasmic reticulum (SR) via ryanodine receptors (RyR2). After Ca2+-induced contraction of the sarcomere, myocyte relaxation occurs when calcium ions are pumped back into the SR by sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) or removed from the myocyte by the Na+/Ca2+ exchanger (NCX). Proper structure and function of junctophilin-2 (JPH2) is believed to be essential for proper excitation-contraction coupling in cardiac myocytes.
JPH2 may also modulate Ca2+ handling by direct interactions with Ca2+ channels. In skeletal muscle, JPH1 was shown to bind directly to the skeletal muscle isoform RyR17. It is therefore likely that JPH2 will also bind to RyR2 in cardiac myocytes, although this remains to be confirmed experimentally. In addition, it was shown that JPH2 binds to the canonical-type transient receptor potential cation channel type 3 (TRPC3), although the physiological role of this interaction is still unknown8. Therefore, changes in the expression level or function of JPH2 may impact intracellular Ca2+ handling in various ways. As such, JPH2 may represent an interesting molecular target to normalize disease-induced changes in Ca2+ homeostasis9.
Compromised EC coupling has been postulated as a key cellular mechanism for defective cardiac contractility in failing hearts10. Gomez et al10 elegantly demonstrated that the ability of VGCC to trigger Ca2+ release from the SR via RyR2 (ie, the gain of EC coupling) was reduced in rats with heart failure. Because expression levels of VGCC and RyR2 were normal in these failing hearts, the defect was localized to the coupling between both types of Ca2+ channels. More recently, Xu et al11 proposed that decreased JPH2 expression might underlie defective EC coupling in rats with cardiac hypertrophy. Decreased JPH2 expression has also been reported in two mouse models of heart failure, the muscle-LIM protein knockout model of dilated cardiomyopathy and the activated H-ras transgenic mouse model of hypertrophic cardiomyopathy5. These results suggest that loss of JPH2 in failing hearts may contribute to defects in EC coupling, although it remains unclear whether JPH2 alterations play a primary or secondary role in the development of heart disease.
Landstrom et al12 recently reported mutations in the JPH2 gene in patients with hypertrophic cardiomyopathy (HCM). Three missense mutations (S101R, Y141H, and S165F) were found in 388 unrelated patients with HCM, but were absent in 500 control individuals. None of the JPH2 mutation carriers had mutations in any other known HCM-linked gene. Matsushita et al13 also reported a mutation in JPH2 (G505S) in a Japanese cohort of HCM patients. Expression of mutant but not wild-type JPH2 in H9c2 cells caused cellular hypertrophy12. Moreover, overexpression of mutant JPH2 in HL-1 cardiomyocytes attenuated the amplitude of Ca2+ transients, suggesting that the EC coupling process was disrupted in cells expressing mutant JPH212. Additional studies will be needed to further characterize the effects of mutant JPH2 in the context of adult cardiac myocytes. Nevertheless, these translational studies suggest that abnormal JPH2 function may lead to hypertrophy and heart failure in patients.
Conclusion
Junctophilin-2 has emerged as a potentially important regulator of excitation-contraction coupling in cardiac myocytes. Although the physiological role of JPH2 needs to be studied more extensively, it is currently believed that JPH2 plays a critical role in properly spacing and aligning VGCCs in the plasma membrane and ryanodine receptors on the sarcoplasmic reticulum. Reduced levels of JPH2 may contribute to defective excitation-contraction coupling in cardiac disease states such as hypertrophic cardiomyopathy and heart failure. Therefore, targeting JPH2 and its binding partners may represent a new therapeutic strategy for the treatment of heart disease.
References
Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K . Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell 2000; 6: 11–22.
Nishi M, Sakagami H, Komazaki S, Kondo H, Takeshima H . Coexpression of junctophilin type 3 and type 4 in brain. Brain Res Mol Brain Res 2003; 118: 102–10.
Garbino A, van Oort RJ, Dixit SS, Landstrom AP, Ackerman MJ, Wehrens XH . Molecular evolution of the junctophilin gene family. Physiol Genomics 2009; 37: 175–86.
Im YJ, Davis AJ, Perera IY, Johannes E, Allen NS, Boss WF . The N-terminal membrane occupation and recognition nexus domain of Arabidopsis phosphatidylinositol phosphate kinase 1 regulates enzyme activity. J Biol Chem 2007; 282: 5443–52.
Minamisawa S, Oshikawa J, Takeshima H, Hoshijima M, Wang Y, Chien KR, et al. Junctophilin type 2 is associated with caveolin-3 and is down-regulated in the hypertrophic and dilated cardiomyopathies. Biochem Biophys Res Commun 2004; 325: 852–6.
Wehrens XH, Lehnart SE, Marks AR . Intracellular calcium release and cardiac disease. Annu Rev Physiol 2005; 67: 69–98.
Phimister AJ, Lango J, Lee EH, Ernst-Russell MA, Takeshima H, Ma J, et al. Conformation-dependent stability of junctophilin 1 (JP1) and ryanodine receptor type 1 (RyR1) channel complex is mediated by their hyper-reactive thiols. J Biol Chem 2007; 282: 8667–77.
Woo JS, Hwang JH, Ko JK, Kim do H, Ma J, Lee EH . Glutamate at position 227 of junctophilin-2 is involved in binding to TRPC3. Mol Cell Biochem 2009; 328: 25–32.
Santonastasi M, Wehrens XH . Ryanodine receptors as pharmacological targets for heart disease. Acta Pharmacol Sin 2007; 28: 937–44.
Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, et al. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science 1997; 276: 800–6.
Xu M, Zhou P, Xu SM, Liu Y, Feng X, Bai SH, et al. Intermolecular failure of L-type Ca2+ channel and ryanodine receptor signaling in hypertrophy. PLoS Biol 2007; 5: e21.
Landstrom AP, Weisleder N, Batalden KB, Bos JM, Tester DJ, Ommen SR, et al. Mutations in JPH2-encoded junctophilin-2 associated with hypertrophic cardiomyopathy in humans. J Mol Cell Cardiol 2007; 42: 1026–35.
Matsushita Y, Furukawa T, Kasanuki H, Nishibatake M, Kurihara Y, Ikeda A, et al. Mutation of junctophilin type 2 associated with hypertrophic cardiomyopathy. J Hum Genet 2007; 52: 543–8.
Acknowledgements
Xander HT WEHRENS is a WM Keck Foundation Distinguished Young Scholar in Medical Research, and is supported by NIH/NHLBI grants R01-HL089598 and R01-HL091947, Muscular Dystrophy Association grant #69238, and the Fondation Leducq Award to the “Alliance for Calmodulin Kinase Signaling in Heart Disease”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garbino, A., Wehrens, X. Emerging role of junctophilin-2 as a regulator of calcium handling in the heart. Acta Pharmacol Sin 31, 1019–1021 (2010). https://doi.org/10.1038/aps.2010.116
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.116
Keywords
This article is cited by
-
Morphogenesis of T-tubules in heart cells: the role of junctophilin-2
Science China Life Sciences (2013)