Abstract
Aim:
The present study determined the extent to which individual differences in responses to the psychostimulating effect of nicotine during adolescence predict similar individual differences during adulthood in rats. We also examined the possible long-term effects of adolescent nicotine exposure on adult prepulse inhibition (PPI) of the acoustic startle response, a measure of sensorimotor gating ability.
Methods:
During the adolescent phase, rats were administered saline, 0.10, 0.40, or 0.60 mg/kg nicotine via subcutaneous injections for 8 days, and motor activity was measured daily. During the adult phase, these rats were treated with the same nicotine dose as in adolescence for 8 additional days. The adolescent saline rats (now adults) were subdivided into four groups and administered saline, 0.10, 0.40, or 0.60 mg/kg nicotine, respectively. PPI was assessed 12 days after the last nicotine treatment.
Results:
During both phases, nicotine increased motor activity across test days in a dose-dependent manner. Motor activity of rats treated with nicotine during adolescence was positively correlated with the activity recorded from the same rats during adulthood. In both phases, there were profound individual differences in the responses to the nicotine treatments. In addition, adolescent rats treated with nicotine did not show decreased motor response to the initial exposure to nicotine. Finally, adolescent exposure to nicotine at 0.4 mg/kg, but not adulthood exposure to the same dose of nicotine, produced a robust disruption of PPI, with individual rats showing different degrees of PPI disruption.
Conclusion:
These findings suggest that adolescent rats have increased sensitivity to the psychostimulating effect and decreased sensitivity to the aversive effect of nicotine. Also, nicotine exposure during adolescence may have long-term detrimental effects on sensorimotor gating ability.
Similar content being viewed by others
Introduction
Several lines of evidence from epidemiological studies suggest that, compared with adults, adolescents may have enhanced vulnerability to nicotine use1. After their initial experience with tobacco, adolescents report fewer aversive effects (eg, nausea, coughs, and dizziness) and more positive effects (eg, euphoria, heightened arousal and attention, reduced stress and anxiety) than adults2. Persons who start smoking as adolescents also experience more difficulty quitting than those who start as adults3. Finally, there is also evidence suggesting that the earlier an individual starts smoking, the more likely he/she will become a lifelong smoker4.
Research in animal models is in agreement with these clinical observations. Adolescent rats are more sensitive to the positive rewarding effects of nicotine and less sensitive to its negative aversive effects than adult rats. With regard to the positive effects of nicotine, adolescent female rats acquire intravenous nicotine self-administration more rapidly than adults5. They also show a higher self-administration rate during both adolescent and adult periods relative to rats that initiate nicotine self-administration as adults6, 7. In addition, administration of relatively low doses of nicotine results in place preference in adolescents but not in adults8, 9, 10. With regard to the aversive effects of nicotine, O'Dell et al (2004) found that adolescent rats displayed fewer somatic signs of nicotine withdrawal (eg, eye blinks, body and head shakes, ptosis, teeth chattering, and yawns) than adult rats, even when blood levels of nicotine were equivalent in both groups11. Moreover, in an elevated plus maze test, Wilmouth and Spear observed that adolescent rats displayed less anxiety-like behaviors than adults following nicotine withdrawal12.
Despite enhanced vulnerability, only a small percentage of the people who start smoking in adolescence become addicted to nicotine. In the US, more than 60% of young people try smoking, but only about one-third to one-half of them become daily smokers13. This clearly suggests that there are marked individual differences in susceptibility to nicotine addiction. Clinical studies suggest that psychosocial factors, such as peer and parental influences14 and behavioral characteristics associated with adolescence, including risk taking, novelty seeking, and impulsivity15, 16, are likely contributing factors to an individual's vulnerability to drug abuse. However, preclinical work aimed at elucidating the neurobiological and behavioral underpinnings of such individual vulnerability is still lacking.
In the present study, we examined to what extent individual differences in the behavioral response to the psychostimulating effect of nicotine during adolescence predict the similar individual differences observed in adulthood. Motor activity is a well-established measure of the psychostimulating effect of nicotine and has been used in both adolescent and adult rats17, 18, 19. We recorded rat motor activity in response to nicotine treatment at both the adolescent and adult phases. We then examined the possible correlations between these two sets of data. We also examined the individual vulnerability to the possible detrimental effects of adolescent nicotine exposure on adult cognitive functions. To this end, we measured the prepulse inhibition (PPI) of the acoustic startle response in adult rats that had been exposed to different doses of nicotine treatment during adolescence and/or during adulthood. PPI is a measure of the reduction in the startle response to a strong acoustic stimulus when that stimulus is shortly preceded by a weaker prepulse stimulus. Specifically, PPI assesses sensorimotor gating, the neural process controlling the integration and processing of sensory information, which is usually thought of as a pre-attentive filtering mechanism. Nicotine-dependent adolescent rats, but not adult rats, show impairment in PPI upon nicotine withdrawal12. To date, no study has examined the long-term negative consequences of adolescent nicotine exposure on PPI in adult rats and the variations in the sensitivity to this detrimental effect across individuals.
Materials and methods
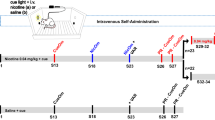
Animals The subjects were 79 Sprague-Dawley rats (42 males and 37 females) from 10 litters (7–8 rats from each litter). The dams were purchased from Charles River Inc. (Portage, MI) on gestation days 13–15. After arrival, the pregnant rats were housed individually, in plastic tubs lined with aspen shavings in a colony on a 12-h light-dark cycle (lights on at 6:30 am). The temperature in the humidity-controlled colony was maintained at approximately 23 °C. Starting one or two days before the first possible expected parturition date (gestation days 22–23), the pregnant females were monitored every morning for signs of parturition. Once the dams were found with pups in the morning (the day designated postnatal day 1, PND 1), each litter was culled to 8 pups (4 males and 4 females with the most visible milk bands). The dams and their litters were housed together for 22 days, after which the pups were weaned from their mothers and housed 4 per cage (same-sex littermates). At PND 45, the pups were separated and housed in same-sex pairs for the remainder of the experiment. All animal procedures were conducted in accordance to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Nebraska Institutional Animal Care and Use Committee.
Locomotor activity recording apparatus Five identical two-compartment chambers custom designed and manufactured by Med Associates (St Albans, VT) were used for the experiments. Each box was housed in a ventilated, sound-insulated isolation cubicle (96.52 cm wide×35.56 cm deep×63.5 cm high). Each box was 64 cm long, 30 cm high (from grid floor), and 24 cm wide and was divided into two equal-sized compartments by a partition with an arch style doorway (15 cm high×9 cm wide at base) and a 4 cm high barrier. The grid floor consisted of 40 stainless-steel rods (0.48 cm diameter), spaced 1.6 cm apart center to center. Below the floor was a stainless steel tray used to collect urine and feces. Illumination was provided by two houselights mounted at the top of each compartment. Activity was recorded by a set of 16 photobeams (ENV-256-8P, 3.175 cm center-to-center) affixed at the bottom of the box (3.5 cm above the grid floor) and controlled by Med Associates computer programs. Background noise (74 dB) was provided by a ventilation fan affixed at the top corner of each isolation cubicle.
Prepulse inhibition of acoustic startle reflex apparatus All prepulse inhibition testing was performed using six Startle Monitor Systems (Kinder Scientific, Julian, CA). Each system, controlled by a PC, was housed in a compact sound attenuation cabinet (35.56 cm wide×27.62 cm deep×49.53 cm high). A speaker (diameter: 11 cm) mounted on the cabinet's ceiling was used to generate acoustic stimuli (70 dB–120 dB). The startle activity was measured by a piezoelectric sensing platform on the floor. During testing, rats were placed in a rectangular box made of transparent Plexiglas (19 cm wide×9.8 cm deep×14.6 cm high) with an adjustable ceiling positioned atop the box, providing only limited restraint while prohibiting ambulation.
Drugs Doses of nicotine hydrogen tartrate (Sigma, St Louis) are expressed as free base dissolved in 0.9% saline. The nicotine solution was brought to a pH of 7.0±0.2 with NaOH. For adolescent rats (PND 28–42)15, 30-gauge needles were used to minimize animal discomfort during injections; 26.5-gauge needles were used for adults.
Experimental groups Seventy-nine Sprague-Dawley rats (42 males and 37 female) were used in this experiment and were assigned to seven groups. Each group consisted of 1 male and 1 female from different litters, with a total of 8-12 subjects (Table 1). Efforts were made to assign an equal number of males and females from each litter to each treatment group.
Experimental procedure After 2 days of habituation to the rectangular two-compartment boxes and to the needle injections, all subjects (PNDs 28−35) were injected with one of three doses of nicotine (0.1, 0.4, 0.6 mg/kg, sc) or saline, and they were immediately placed in the testing boxes where motor activity was measured for 30 min. Once the subjects became adults (PND 70–71), handling was resumed (1 min/d). From PND 72 to 79, rats were injected with their corresponding solution (saline, 0.1, 0.4, or 0.6 mg/kg nicotine, sc), and their motor activity was tested (once daily). Rats exposed to nicotine as adolescents received the same dose of nicotine as adults. A subset of the rats that had been exposed to saline as adolescents received nicotine for the first time as adults. As denoted in Table 1, this protocol resulted in 7 experimental groups (Table 1). Lastly, on PND 80, all rats were injected with the same dose of nicotine (0.4 mg/kg, sc), and motor activity was recorded for 30 min. This test examined the long-term behavioral sensitization effect of repeated nicotine exposure during the adolescent and adult phases using a common nicotine dose.
On PND 92 to 96 (12 days after the last adult nicotine treatment), rats were tested daily for PPI across five consecutive days20. In the first two test days (PND 92–93, Baseline tests), rats were placed individually into the PPI boxes and exposed to 5 min of 70-dB white background noise, which continued throughout the entire testing session. The initial 5 min was followed by 32 trials consisting of two different protocols presented in pseudorandom order: 17 “PULSE ALONE” trials, each consisting of a 40 ms 120-dB noise burst (the 'pulse'), and 15 “PREPULSE+PULSE” trials consisting of a 20 ms noise burst of 73, 76, or 82 dB followed 100 ms later by the 120-dB pulse (5 trials at each dB level). Startle magnitude was defined as the maximum force (measured in Newtons) applied by the rat to the startle apparatus during a period of 100 ms after the onset of the pulse stimulus. During the following three days (PND 94–96), a slightly different PPI testing procedure was used based on a previously reported protocol20. Each test session consisted of five different trial types: PULSE ALONE trials (n=18), three types of PREPULSE+PULSE trials (n=30, 10 trials/type) identical to the ones run during baseline, and new split 76 dB trials, which consisted of two 20 ms 76 dB prepulses separated by 10 ms, followed 10 ms later by the 120 dB pulse (n=10). The first and last four trials in each PPI testing session were of the PULSE ALONE type. All remaining trials were presented in pseudorandom order and were separated by a variable inter-trial interval (mean 15 s, ranging from 9–21 s).
Data analysis Data from the adolescent and adult phases were first analyzed separately using repeated-measures analyses of variance (ANOVAs) with a within-subjects factor of test days and between-subjects factors of nicotine treatment and sex. Post hoc Tukey's HSD tests were used to determine group differences. To examine how early adolescent nicotine treatment impacts the effects of nicotine in adult rats, data from the groups exposed to nicotine at both the adolescent and adult phases were compared with data of the groups exposed to the same treatment only at the adult phase. In addition, linear regression tests were employed to estimate the correlation between the motor measurements obtained at the adolescent and adult phases.
For the PPI data, startle responses in the PULSE and PREPULSE+PULSE trials were used to calculate percent prepulse inhibition (%PPI) using the following equation:
%PPI=100–[(mean startle response to PREPULSE+ PULSE trials/mean startle response to PULSE ALONE trials)*100]
We compared each pair of nicotine groups (eg, Nic-Nic-0.1 mg/kg and Sal-Nic-0.1 mg/kg) with the saline group using repeated measures ANOVA with the nicotine treatment as a between-subjects factor, and test days and levels of PPI as within-subject factors. If a significant nicotine treatment effect was detected, one-way ANOVA was used to examine the exact differences at the specific PPI level and test days.
Results
Effect of nicotine treatment on motor activity during adolescence Overall, there was a significant effect of “Sex” during adolescence (F(1, 65)=9.57, P=0.003) and adulthood (F(1, 65)=28.07, P<0.001). The females were generally more active than the males, which was consistent with previous reports9, 17. There were no significant interactions between “Sex” and other factors (eg, days and treatment) (Ps>0.40); therefore, data were combined for male and female subjects for the rest of the analysis.
As shown in Figure 1A, during the adolescent phase, nicotine increased motor activity progressively and in a dose-dependent manner. This effect tapered off toward the last two test days. Repeated measures ANOVA indicated that there was a significant effect of nicotine treatment (F(6,72)=29.75, P<0.001), test days (F(7,504)=9.409, P<0.001) and a significant interaction between nicotine and test days (F(42, 504)=11.741, P<0.001). Post hoc Tukey tests indicated that the nicotine 0.4 and 0.6 mg/kg groups were significantly different from the nicotine 0.1 mg/kg group and the four saline groups (Ps≤0.005) but did not differ from each other. The nicotine 0.1 mg/kg group was also significantly different from the four saline groups (Ps≤=0.010), which were not significantly different from each other (all Ps>0.97). One-way ANOVAs on each of the 8 test days showed that there were no group differences on the first day of testing (F<1), but differences did appear by the second day of testing (P<0.001). More importantly, there were substantial individual differences in motor activity among the nicotine-treated adolescents (≥2-fold), even among rats treated with the same dose of nicotine. Figure 1B shows an example of such data from the nicotine 0.4 mg/kg group.
(A) Motor activity (beam breaks) (group means±SEM) for the adolescent rats treated with nicotine 0.1, 0.4, and 0.6 mg/kg or saline from PND 28 to 35 and tested each day for 30 min. (B) Motor activity for the individual adolescent rats from the nicotine 0.4 mg/kg group over the 8 test days. bP<0.05 vs Sal-Sal group.
Effect of nicotine treatment on motor activity during adulthood During the adult phase, nicotine also increased motor activity progressively over successive test days (Figures 2A and 2B). Repeated measures ANOVA revealed a significant effect of nicotine treatment (F(6,72)=3.393, P=0.005), test days (F(7,504)=97.227, P<0.001) and an interaction between nicotine and test days (F(42, 504)=6.632, P<0.001). Prior adolescent nicotine exposure also affected adult responses to nicotine, as there were differences between the rats that had been exposed to nicotine during adolescence versus those that only received nicotine during adulthood (see the circled data points in Figures 2A and 2B). Among rats that had been exposed to nicotine during adolescence, activity levels increased progressively with time (Day: F(7, 280)=34.88, P<0.001; Treatment: F(3, 40)=3.422, P=0.026; Day×Treatment interaction: F(21, 280)=8.433, P<0.001). The differences between one of the nicotine treatment groups and the saline group started to appear after four days of treatment and persisted throughout the remaining test days (all Ps<0.026). Among rats first exposed to nicotine as adults, motor activity was initially suppressed in a dose-dependent manner (F(3, 42)=12.06, P<0.001). With repeated treatment, activity in the nicotine groups increased in a dose-dependent fashion and eventually became higher than that of the saline controls. The 0.4 mg/kg (but not 0.1 or 0.6 mg/kg) group was significantly different from the saline group (Day 5−8: Ps<0.003). The finding that the nicotine's initial suppressive effect was absent in the adolescent nicotine rats suggests that adolescent rats are less sensitive to the aversive and unpleasant effects of nicotine.
(A) Motor activity (beam breaks) (group means±SEM) for the adult rats that were previously treated with nicotine 0.1, 0.4 and 0.6 mg/kg or saline during adolescence and were retested with the same nicotine treatment during adulthood for 8 days. (B) Motor activity (# beam breaks) (group means±SEM) for the adult rats that were treated with saline during adolescence, and were tested with nicotine 0.1, 0.4 and 0.6 mg/kg or saline daily for 30 min during adulthood (8 days of treatment). bP<0.05 vs Sal-Sal group.
Comparison of rats that had been exposed to nicotine during adolescence to those that only received nicotine during adulthood further indicated that adolescent nicotine exposure altered adult motor responses to nicotine in a dose-dependent manner. For the 0.1 mg/kg groups (Figures 3A), there was a significant effect of Test (F(1, 154)=11.25, P<0.001), but no significant effect of Group or Group× Test interaction (Fs≤1.215, Ps≥0.298). For the 0.4 mg/kg groups (Figure 3B), there was a significant effect of Test (F(1, 154)=89.179, P<0.001) and a significant Group×Test interaction (F(7,154)=2.170, P=0.040); the main effect of Group was not significant (F<1). The adolescent nicotine (0.4 mg/kg) group had higher motor activities at the early (day 1−3) and late (day 5−8) test days than the adult nicotine (0.4 mg/kg) group. For the 0.6 mg/kg groups (Figure 3C), however, there was a significant effect of Test (F(1, 147)=44.99, P<0.001), a significant effect of Group (F(1,21)=8.719, P=0.008), but no significant interaction (F<1). The rats exposed to 0.6 mg/kg nicotine as adolescents displayed consistently higher motor activities than the adult nicotine group throughout the entire test period. Similar to what was observed in adolescent rats (Figure 1B), there were also large individual differences in the motor response to nicotine treatment in adult rats. Figure 3D depicts the motor activity of individual rats in the nicotine-nicotine (0.4 mg/kg) group during adulthood.
(A) Motor activity (beam breaks) (group means±SEM) for the adult rats that were previously treated with nicotine 0.1 mg/kg or with saline during adolescence and were later tested with nicotine 0.1 mg/kg during adulthood for 8 days. (B) Motor activity (beam breaks) (group means±SEM) for the adult rats that were previously treated with nicotine 0.4 mg/kg or with saline during adolescence and were tested with nicotine 0.4 mg/kg during adulthood for 8 days. bP<0.05 vs Sal-Nic 0.4 mg/kg group. (C) Motor activity (beam breaks) (group means±SEM) for the adult rats that were previously treated with nicotine 0.6 mg/kg or with saline during adolescence and were tested with nicotine 0.6 mg/kg during adulthood for 8 days. bP<0.05 vs Sal-Nic 0.6 mg/kg group. (D) Motor activity for the individual adult rats from the Nic-Nic 0.4 mg/kg group over the 8 test days. (E) Motor activity (beam breaks) (group means±SEM) for the 7 groups of adult rats in the final nicotine (0.4 mg/kg, sc) test. bP<0.05 vs Sal-Sal group; eP<0.05 vs Sal-Nic 0.1 mg/kg and Nic-Nic 0.1 mg/kg groups.
The last nicotine injection was tested for behavioral sensitization to nicotine. To this end, all rats were injected with the same dose of nicotine (0.4 mg/kg, sc) and tested for 30 min. We found that rats that had been previously treated with nicotine (either during adolescence or adulthood) showed significantly higher motor activities than the saline group rats, indicating a robust sensitization effect (F(6,78)=29.106, P<0.001) (Figure 3E). Prior adolescent nicotine treatment did not significantly potentiate the nicotine-induced sensitization effect, as there were no significant differences between the groups that received nicotine during both adolescence and adulthood and the groups that received nicotine only during adulthood (all P>0.05).
Effect of early adolescence nicotine treatment on PPI during adulthood To examine the long-term effect of adolescent nicotine exposure on the rats' cognitive functions, we assessed the PPI from PND 92 to 96 (12 days after the last adult nicotine treatment) daily for 5 consecutive days. One rat from the nicotine-nicotine 0.6 mg/kg group died unexpectedly, leaving only 10 rats from that group to be tested for PPI. Figure 4 shows the mean percentage PPIs (prepulses: 3, 6, and 12 dB above background) for the 6 nicotine groups, each plotted together with the saline control group. For the two 0.1 mg/kg groups (Figure 4A), repeated measures ANOVA with the nicotine treatment as a between-subject factor and test days and levels of PPI as within-subject factors, showed that there was a significant effect of test days (F(4, 116)=32.180, P<0.001) and a significant effect of levels (F(2,58)=145.444, P<0.001), but no significant effect of nicotine treatment or any interaction involving nicotine treatment and other factors (all Ps>0.05). This suggests that this concentration of nicotine (0.1 mg/kg) given during adolescence and adulthood did not alter PPI significantly.
The long-term effect of adolescent nicotine exposure on PPI. Rats were tested in two different PPI procedures (Baseline day 1 and 2, and PPI day 3–5, see text for details). (A) PPIs for the two nicotine 0.1 mg/kg groups (Nic-Nic 0.1 mg/kg and Sal-Nic-0.1 mg/kg) and the saline control group over the 5 test days. Prepulses were 3, 6 or 12 dB above background (70 dB). (B) PPIs for the two nicotine 0.4 mg/kg groups (Nic-Nic 0.4 mg/kg and Sal-Nic-0.4 mg/kg) and the saline control group over the 5 test days. Prepulses were 3, 6 or 12 dB above background (70 dB). Asterisks indicate significant differences from the saline group. (C) PPIs for the two nicotine 0.6 mg/kg groups (Nic-Nic 0.6 mg/kg and Sal-Nic-0.6 mg/kg) and the saline control group over the 5 test days. Prepulses were 3, 6 or 12 dB above background (70 dB). (D) 76 dB PPIs for the individual adult rats from the Nic-Nic 0.4 mg/kg group over the 5 test days. bP<0.05 vs Sal-Sal groups. eP<0.05 vs Sal-Nic groups.
For the two 0.4 mg/kg groups (Figure 4B), repeated measures ANOVA showed that there were significant effects of test days (F(4, 116)=33.913, P<0.001), PPI levels (F(2,58)=178.968, P<0.001), and nicotine treatment (F(2,29)=4.094, P=0.027), as well as a significant three-way interaction among these factors (F(16,232)=1.901, P=0.021). One-way ANOVA followed by post hoc Tukey's test indicated that rats that treated with 0.4 mg/kg nicotine at both adolescent and adult phases exhibited significantly lower PPIs than the rats that were only treated with nicotine during the adult phase and the saline control rats. This disruptive effect of adolescent nicotine treatment was more conspicuous on days 4 and 5.
For the two 0.6 mg/kg groups (Figure 4C), repeated measures ANOVA found a significant effect of test days (F(4, 108)=27.893, P<0.001) and a significant effect of levels (F(2,54)=213.192, P<0.001). There was no effect of nicotine treatment or any interaction involving nicotine treatment and other factors (all Ps>0.05), suggesting that nicotine 0.6 mg/kg, like nicotine 0.1 mg/kg, also did not significantly disrupt PPI when the drug was given during adolescence.
To illustrate the individual vulnerability to this disruptive effect, we plotted the 76 dB PPI data from all rats in the Nic-Nic 0.4 mg/kg group. As can be seen in Figure 4D, there were substantial differences in the PPI performance across individuals.
Correlation analysis of motor activity measured at both phases We used linear regression to examine the correlation between motor activity in the adolescent and adult phase. Figure 5 shows motor activity averaged over the 8 drug days (group means±SEM) for individual adolescent and adult nicotine-treated rats. These two sets of data were highly correlated (Pearson correlation r=0.548, P=0.001). The linear regression equation was the following: Y (adult) =1347.91+0.94*x (adolescent). r2=0.301. The coefficient was also significant (F(1, 34)=14.61, P=0.001), suggesting that the individual differences in the responses to nicotine that were present during adolescence persisted through to adulthood. Thus, it may be possible to predict adult motor activity based on motor activity recorded during adolescence.
We next examined whether there was any relationship between an individual rat's motor response to nicotine during adolescence and its PPI performance during adulthood. To this end, we used the Bivariate Correlations procedure and computed the Pearson's correlation coefficient between the motor activity data obtained during adolescence in adolescent nicotine-treated rats and their PPI data recorded during the adult phase. There were no significant correlations between these two sets of data (all Ps>0.05), suggesting that the individual differences in motor response to nicotine during adolescence do not directly predict sensorimotor gating in adulthood.
Discussion
The present study demonstrates that individual differences in the motor responses to the psychostimulating effect of nicotine during adolescence are positively correlated with the differences seen during adulthood. This suggests that individual sensitivity to the effects of nicotine, or even susceptibility to nicotine abuse, may be detected during the early adolescent period. We also found different patterns of motor responses to nicotine between adolescent and adult rats. Both adolescent and adult rats showed a dose-dependent increase in motor responses to the repeated nicotine treatment. However, the adolescent nicotine-treated rats were less sensitive to the initial motor suppressive effect or aversive effect of nicotine exposure than adult rats. Specifically, the adolescent nicotine-treated rats did not show decreased motor activity on the first 2 days of nicotine treatment, whereas adult rats did. Early adolescent nicotine exposure also abolished the motor-depressing effect in adult rats that had been treated with nicotine during adolescence. Adolescent nicotine exposure significantly potentiated later adult motor response to nicotine, such that adolescent nicotine exposure rats showed significantly higher motor activity than those that only received nicotine during adulthood. Finally, we found that adolescent exposure to nicotine 0.4 mg/kg, but not to 0.1 and 0.6 mg/kg, caused a disruption in the PPI, and that there were large individual differences in PPI performance.
Adolescents as well as adults exhibited a progressively enhanced motor response (eg, sensitization) to nicotine's activity-increasing action over the 8 days of drug treatment (see Figures 1A and 2A and 2B). This sensitization effect was further confirmed in the final nicotine test, which showed that rats that had been previously treated with nicotine had significantly higher motor activity levels than the saline control rats (Figure 3E). Of interest is the finding that there were no significant differences in sensitization between the groups that were exposed to nicotine only during adulthood and those that were exposed to nicotine during both developmental phases. This lack of long-term behavioral sensitization of adolescent nicotine exposure may be due to a ceiling effect. The challenge test was conducted after 8 consecutive days of nicotine treatment during adulthood, and any possible adolescent nicotine sensitization effect might have been masked by the adult nicotine treatment. These activity patterns are consistent with data reported in the literature17, 21, 22, 23. We also found that adolescent rats might be less sensitive to the initial aversive effect of nicotine than adult rats, as adolescent rats did not show decreased motor activity on the first 2 days of nicotine treatment, whereas adult rats did. This initial motor suppressing effect has been linked to the aversive effect of nicotine that tolerates out rather rapidly across-species23. This finding is consistent with the results from Vastola et al9, who showed that, relative to adults, adolescent rats were less sensitive to the nicotine's motor-suppressing effect and more sensitive to the psychomotor effect of nicotine. More interestingly, early adolescent nicotine exposure completely blocked this acute motor-suppressing effect of nicotine in adult rats (Figure 2A).
As mentioned in the Introduction, previous work has shown that adolescent rats are less sensitive to nicotine withdrawal. Specifically, they often display fewer somatic signs of nicotine withdrawal than adult rats11, they fail to develop a conditioned place aversion induced by mecamylamine-precipitated nicotine withdrawal24, and they display less anxiety-like behaviors following nicotine withdrawal12. Our finding that adult rats that had been exposed to nicotine during adolescence also showed less sensitivity to the nicotine's motor suppressive effect during adulthood adds to this literature. To the extent that the negative effects of nicotine and nicotine withdrawal play a crucial role in the maintenance of long-term nicotine use25, our finding suggests that adolescent nicotine exposure may have persistent effects leading to more tenacious nicotine addiction in adults.
The finding that early adolescent nicotine exposure enhanced motor responses to adult nicotine treatment is also consistent with what has been reported in the literature. For example, Faraday et al reported that male rats first exposed to nicotine as adolescents exhibit greater sensitivity to the motor stimulating effect of nicotine when they are retested during adulthood compared with rats that are exposed to nicotine for the first time during adulthood18. Elliot et al found a similar effect in female rats17.
In this study, we also found that rats that were treated with nicotine 0.4 mg/kg during adolescence and adulthood, but not rats that were treated with the same dose of nicotine only during adulthood, showed impaired PPI when they were assessed during the abstinence period (12 days after the last nicotine treatment) relative to the saline rats (Figure 4B). We also observed large individual differences in PPI performance over the 5 test days (Figure 4D). Previous studies have found that nicotine increases PPI in Sprague-Dawley adult rats, but not in adolescent rats26, 27, 28, whereas nicotine withdrawal generally has no apparent effect on PPI in adult rats27, 28 but causes an acute disruption of PPI in adolescent rats12. We are not aware of any study that has examined the long-term effects of adolescent nicotine exposure on PPI in adult rats and the individual sensitivity to this detrimental effect. The PPI deficit observed in rats that were exposed to nicotine 0.4 mg/kg at both adolescent and adulthood phases could be attributed to two possible sources. The first source would be the exposure to nicotine during adolescence, while the second one would be the two prior exposures to nicotine. Because we did not have a group that was treated with 0.4 mg/kg nicotine only during adolescence, it is impossible to identify which of these two sources was responsible for the PPI deficit. However, the finding that rats exposed to nicotine only during the adulthood phase did not show a PPI deficit strongly suggests that adolescent nicotine exposure is critical in causing the PPI deficit. This point is also supported by a study by Wilmouth and Spear, which reports that PPI was significantly disrupted in adolescent rats previously exposed to nicotine, but not in adult rats12.
The underlying mechanisms that support such a long-term effect of adolescent nicotine exposure on adult PPI remain unclear. The mesolimbic dopamine system is thought to be critically involved in PPI29, and this system undergoes developmental changes during adolescence and overlaps with the neural circuitry regulating the positive and psychostimulating effects of nicotine and nicotine withdrawal15. Therefore, we speculate that adolescent nicotine exposure may permanently derail the developmental trajectory of the mesolimbic dopamine system in a way that leads to impaired cognitive functioning. Indeed, previous work has shown that rats exposed to nicotine during adolescence show increased catecholamine (eg, norepinephrine and dopamine) turnover during the treatment period, a drop in midbrain catecholamine turnover upon immediate nicotine withdrawal, and a later-emerging activation of these pathways during adulthood30. Adolescent rats exposed to nicotine also show an upregulation of nicotinic receptors31 and an increase in nicotinic acetylcholine receptor gene expression32. It should be noted that other neurochemical systems that are not directly involved in the regulation of PPI and nicotine effects could also be negatively affected by adolescent nicotine exposure. This point is supported by the lack of significant correlation between an individual rat's motor response to nicotine during adolescence and its PPI performance during adulthood. This suggests that neural systems other than those involved in regulating the positive and psychostimulating effects of nicotine and nicotine withdrawal may contribute to the adolescent nicotine-induced PPI disruption. It would be valuable for future research to comprehensively evaluate the cognitive functions and emotional regulation of rats that were exposed to nicotine during adolescence and to determine the possible neural and neurochemical mechanisms of the effects of nicotine on adolescents.
In summary, the present study shows that individual adolescent rats show different sensitivity to the psychostimulating effect of nicotine, and these differences observed during adolescence correlate positively with the differences seen during adulthood. Early adolescent nicotine exposure enhances the motor responses to nicotine and blocks the motor-depressing effect in adult rats. Adolescent nicotine exposure also causes PPI disruption in adult rats, and individual rats show different degrees of vulnerability to this adverse effect of nicotine. We conclude that individual differences in sensitivity to the rewarding and aversive effects of nicotine during adolescence may play a critical role in determining nicotine addiction during adulthood.
Author contribution
Dr Ming LI and Dr Rick A BEVINS designed research; Ms Alexa MEAD performed research and collected data; Dr Ming LI analyzed data and wrote the first draft of the paper. Dr Ming LI and Dr Rick A BEVINS wrote the final version of the paper.
References
Barron S, White A, Swartzwelder HS, Bell RL, Rodd ZA, Slawecki CJ, et al. Adolescent vulnerabilities to chronic alcohol or nicotine exposure: findings from rodent models. Alcohol Clin Exp Res 2005; 29: 1720–5.
DiFranza JR, Rigotti NA, McNeill AD, Ockene JK, Savageau JA, St Cyr D, et al. Initial symptoms of nicotine dependence in adolescents. Tob Control 2000; 9: 313–9.
Chen J, Millar WJ . Age of smoking initiation: implications for quitting. Health Rep 1998; 9: 39–46(Eng); 39–48(Fre).
Breslau N, Peterson EL . Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health 1996; 86: 214–20.
Chen H, Matta SG, Sharp BM . Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology 2007; 32: 700–9.
Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS . Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 2003; 169: 141–9.
Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, et al. Adolescent vs adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol 2007; 29: 458–65.
Torrella TA, Badanich KA, Philpot RM, Kirstein CL, Wecker L . Developmental differences in nicotine place conditioning. Ann N Y Acad Sci 2004; 1021: 399–403.
Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP . Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav 2002; 77: 107–14.
Shram MJ, Funk D, Li Z, Le AD . Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006; 186: 201–8.
O'Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF . Nicotine withdrawal in adolescent and adult rats. Ann N Y Acad Sci 2004; 1021: 167–74.
Wilmouth CE, Spear LP . Withdrawal from chronic nicotine in adolescent and adult rats. Pharmacol Biochem Behav 2006; 85: 648–57.
Henningfield JE, Moolchan ET, Zeller M . Regulatory strategies to reduce tobacco addiction in youth. Tob Control 2003; 12 Suppl 1: i14–24.
Simons-Morton B, Haynie DL, Crump AD, Eitel SP, Saylor KE . Peer and parent influences on smoking and drinking among early adolescents. Health Educ Behav 2001; 28: 95–107.
Spear LP . The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 2000; 24: 417–63.
Kelley AE, Schochet T, Landry CF . Risk taking and novelty seeking in adolescence: introduction to part I. Ann NY Acad Sci 2004; 1021: 27–32.
Elliott BM, Faraday MM, Phillips JM, Grunberg NE . Adolescent and adult female rats differ in sensitivity to nicotine's activity effects. Pharmacol Biochem Behav 2005; 80: 567–75.
Faraday MM, Elliott BM, Phillips JM, Grunberg NE . Adolescent and adult male rats differ in sensitivity to nicotine's activity effects. Pharmacol Biochem Behav 2003; 74: 917–31.
Bevins RA, Besheer J . Individual differences in rat locomotor activity are diminished by nicotine through stimulation of central nicotinic acetylcholine receptors. Physiol Behav 2001; 72: 237–44.
Culm KE, Hammer RP Jr. Recovery of sensorimotor gating without G protein adaptation after repeated D2-like dopamine receptor agonist treatment in rats. J Pharmacol Exp Ther 2004; 308: 487–94.
Clarke PB, Kumar R . The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Br J Pharmacol 1983; 78: 329–37.
Besheer J, Bevins RA . Impact of nicotine withdrawal on novelty reward and related behaviors. Behav Neurosci 2003; 117: 327–40.
Stolerman IP . Inter-species consistency in the behavioural pharmacology of nicotine dependence. Behav Pharmacol 1999; 10: 559–80.
Shram MJ, Siu EC, Li Z, Tyndale RF, Le AD . Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male Wistar rats. Psychopharmacology (Berl) 2008; 198: 181–90.
Koob GF, Le Moal M . Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001; 24: 97–129.
Curzon P, Kim DJ, Decker MW . Effect of nicotine, lobeline, and mecamylamine on sensory gating in the rat. Pharmacol Biochem Behav 1994; 49: 877–82.
Acri JB, Brown KJ, Saah MI, Grunberg NE . Strain and age differences in acoustic startle responses and effects of nicotine in rats. Pharmacol Biochem Behav 1995; 50: 191–8.
Faraday MM, O'Donoghue VA, Grunberg NE . Effects of nicotine and stress on startle amplitude and sensory gating depend on rat strain and sex. Pharmacol Biochem Behav 1999; 62: 273–84.
Swerdlow NR, Braff DL, Geyer MA . Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav Pharmacol 2000; 11: 185–204.
Trauth JA, Seidler FJ, Ali SF, Slotkin TA . Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Res 2001; 892: 269–80.
Trauth JA, Seidler FJ, McCook EC, Slotkin TA . Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res 1999; 851:9–19.
Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, et al. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci 2003; 23: 4712–6
Acknowledgements
This study was funded in part by support from the Nebraska Tobacco Settlement Biomedical Research Development Funds and by a research grant from Nebraska Health and Human Services (2007–2008) to Ming LI. Dr Rick A BEVINS was supported in part by DA018114 during conceptualization of the study and preparation of the manuscript. We thank Mr Tao SUN and Wei HE for technical support for the PPI test.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, M., Mead, A. & Bevins, R. Individual differences in responses to nicotine: tracking changes from adolescence to adulthood. Acta Pharmacol Sin 30, 868–878 (2009). https://doi.org/10.1038/aps.2009.55
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2009.55
Keywords
This article is cited by
-
CDP-choline and galantamine, a personalized α7 nicotinic acetylcholine receptor targeted treatment for the modulation of speech MMN indexed deviance detection in healthy volunteers: a pilot study
Psychopharmacology (2020)
-
An acute dose, randomized trial of the effects of CDP-Choline on Mismatch Negativity (MMN) in healthy volunteers stratified by deviance detection level
Neuropsychiatric Electrophysiology (2015)
-
Effects of intravenous nicotine on prepulse inhibition in smokers and non-smokers: relationship with familial smoking
Psychopharmacology (2013)
-
Nicotine: Alcohol Reward Interactions
Neurochemical Research (2010)