Abstract
Aim:
To analyze the results of idarubicin (IDA)- versus etoposide (VP16)-intensified myeloablative conditioning regimen in allogeneic hematopoietic stem cell transplantation (allo-SCT) for high-risk acute leukemia.
Methods:
From January 2005 to June 2008, 48 consecutive patients (male: n=29; median age: 30 years, range 14–51 years) with high-risk acute leukemia underwent allo-SCT following an IDA- or VP16-intensified conditioning regimen. The conditioning regimens were modified BUCY2 (busulfan+cyclophosphamide) consisting of IDA (15 mg/m2 per day, days -12 to -10) or VP16 (25 mg/kg per day, days -3 to -2) and CY/TBI (cyclophosphamide/total body irradiation) intensified with IDA (15 mg/m2 per day, days -6 to -5) or VP16 (25 mg/kg per day, days -3 to -2) for acute myeloid leukemia and acute lymphoblastic leukemia, respectively.
Results:
Between the two groups, no significant differences in terms of baseline characteristics, incidence of acute or chronic graft-versus-host disease (GVHD) or transplant-related mortality (TRM) (P=0.50) were observed. However, the IDA group demonstrated higher incidences of mucositis and Aspergillus pneumonia (P<0.01 and P=0.03, respectively). For the IDA and VP16 groups, relapse rates were 28% and 50%, respectively (P=0.13). For the same groups, the 2-year probabilities of leukemia-free survival (LFS) and overall survival (OS) were 72% versus 51% (P=0.04) and 74% versus 53% (P=0.04), respectively.
Conclusion:
This retrospective analysis suggests that conditioning regimens intensified with IDA can achieve better outcomes than conditioning regimens with VP16 in patients preparing to undergo allo-SCT for high-risk acute leukemia.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic stem-cell transplantation (allo-SCT) is an effective, potentially curative therapy in a variety of hematological malignancies1, 2. Regimens of total body irradiation (TBI) or busulphan (BU) combined with cyclophosphamide (CY) have long been the classical conditioning regimens used worldwide to prepare patients to receive allo-SCT. The overall goals of conditioning regimens are to suppress the immune system, make room in the bone marrow for the donor stem cells to grow, and destroy any residual leukemia cells1, 3. High-risk acute leukemia—including acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) with unfavorable cytogenetics at diagnosis, previous myelodysplastic syndrome (MDS), no complete remission (CR) after the first induction cycle and second or later remission—does not yet have curative options other than allo-SCT4, 5. Therefore, after conventional salvage therapy and achievement of a second complete remission (CR2), most centers proceed to allo-SCT to increase treatment intensity and to exploit a graft-versus-leukemia (GVL) effect. For patients with high-risk acute leukemia undergoing allo-SCT, however, a major obstacle to success is represented by the relapse of the underlying disease and subsequent poor survival6, 7, 8, 9. The main cause for relapse is the fact that the GVL effect is limited by the residual leukemia cell burden, which cannot be effectively eliminated in high-risk acute leukemia10, 11. High-risk acute leukemia patients can benefit from cytoreductive treatment with an intensified regimen before SCT. Additionally, reduced-intensity conditioning (RIC) regimens have been shown to produce durable remissions and long-term disease-free survival in other acute leukemias12.
Although some non-randomized studies on the use of intensified conditioning regimens combined with etoposide (VP16) or idarubicin (IDA) have reported an improved outcome for high-risk acute leukemia patients13, 14, 15, 16, the differences between IDA- and VP16-intensified conditioning regimens with regard to the outcome of these advanced patients remain unclear. Here, we present results obtained at our institute after retrospective comparison of IDA- versus VP-16-intensified conditioning regimens in allo-SCT from January 2005 to June 2008.
Patients and methods
Patients and donors
Forty-eight consecutive AML and ALL patients with high-risk features were treated from January 2005 to June 2008 at the Institute of Hematology, Tongji Medical College, Huazhong University of Science and Technology. The study was approved by the institutional review board of the Union Hospital. Informed consent was obtained from all patients at time of treatment, in accordance with the Declaration of Helsinki. Patients were included if they fulfilled at least one of the following criteria defining high-risk acute leukemia: primary or secondary refractory leukemia (as defined for AML by persisting disease following ≥one course of high-dose cytarabine), extramedullary leukemia, delayed response to induction chemotherapy, relapse within 3 months from induction or consolidation therapy, ≥two relapses, unfavorable cytogenetics17, or acute leukemia secondary to MDS or other malignancies. For ALL, the additional inclusion criteria were age older than 35 years, elevated white blood cell count (WBC) (≥25×109/L), and cytogenetic abnormalities such as t (9;22). Additional inclusion criteria for transplantation were age from 13 to 55 years and the availability of either a related or unrelated stem-cell donor (with ≤one major mismatch). Patients were not eligible for the intensified regimens if they were resistant to IDA or VP16 before the transplant and if they had a severe liver or renal disease, corrected pulmonary diffusion capacity less than 35%, cardiac ejection fraction lower than 40%, Karnofsky performance status less than 80, or any active infection18. AML patients (n=26) were conditioned with the modified BUCY2 regimen that included busulphan (BU) followed by cyclophosphamide (CY) for 2 days, and ALL patients (n=22) were given a conditioning regimen that included CY combined with total-body irradiation (TBI). All conditioning regimens were intensified with etoposide (VP16) (VP16 group, n=20) or idarubicin (IDA) (IDA group, n=28). The selection of VP16 or IDA depended mainly on whether the patient had been resistant to the two drugs previously. Patients previously resistant to VP16 were given IDA, and vice versa. Ten patients received a VP16-intensified regimen for cost considerations — IDA is much more expensive than VP16. Patient characteristics are described in Table 1. All patients were hospitalized for 3 to 4 weeks before being discharged to the outpatient clinic, and all were admitted to the hospital as required for the treatment of complications. Patients and donors were matched for the HLA-A, -B, and -C antigens by serology, whereas intermediate-resolution DNA typing and HLA-DRB1, HLA-DQB1, and HLA-DPB1 typing were performed at the allele level using high-resolution techniques. Patients and donors were matched for the HLA-DRB1 and DQB1 alleles.
Conditioning regimen
For cytoreduction, the conditioning regimen in transplants for AML involved modified BUCY2. The modified BUCY2 with VP16 was administered as follows: hydroxycarbamide (80 mg/kg per day) orally on day -10, cytarabine (2 g/m2 per day) intravenously on day -9, BU (4 mg/kg per day) intravenously on days -8 to -6, CY (1.8 mg/m2 per day) intravenously on days -5 to -4, Me-CCNU (250 mg/kg) orally once on day -3, methylprednisolone (1.5 mg/kg) intravenously once on day -3, and VP16 (25 mg/kg per day) intravenously on days -3 to -2. The modified BUCY2 with IDA consisted of IDA (15 mg/m2 per day) intravenously on days -12 to -10, busulfan (BU, 3.2 mg/kg per day) intravenously on days -6 to -4, and cyclophosphamide (CY, 1.8 mg/m2 per day) and methylprednisolone (1.5 mg/kg per day) intravenously on days -3 to -2; it excluded the management of hydroxycarbamide, cytarabine and Me-CCNU to decrease the risk of excessive cytoreduction. For ALL, the conditioning regimen involved CY/TBI intensified with IDA or VP16. TBI (total body irradiation) was administered at a dose of 8 Gy from a linear accelerator on day -7, and CY was given at a dose of 60 mg/kg once a day on days -3 to -2; additionally, IDA (15 mg/m2 per day) was administered by continuous infusion for more than 20 h on days -6 to -5, or VP16 (25 mg/kg per day) was given intravenously on days -3 to -2.
Mobilization and collection of stem cells
The peripheral blood stem cells (PBSCs) were collected using standard mobilization protocols. Human granulocyte colony-stimulating factor (G-CSF) (5 μg/kg per day) was used to mobilize peripheral blood, and peripheral blood progenitor cells were harvested on day 1 after 5 days of G-CSF. We attempted to reach a target of ≥4×106CD34+ cells or ≥7×108 mononuclear cells (MNCs) to be collected per kilogram of the recipient's body weight. The harvested cells were infused without manipulation on the same day of collection.
Graft-versus-host disease (GVHD) prophylaxis and management
All patients were given a combination of cyclosporine A (CSA), a short course of methotrexate (MTX), and mycophenolate mofetil (MMF) (given only to patients unrelated to their donors). On day +1, MTX (15 mg/m2) was administered intravenously; it was then given at 10 mg/m2 on days +3, +6, and +11 after transplantation. CsA (5 mg/kg twice a day orally) was started on day -1, with trough levels targeted at 150–250 ng/mL during the first 40 days. CsA was then tapered for approximately 60 to 90 days until full discontinuation. MMF (7.5 mg/kg twice a day) was started orally on day +7 in the HLA-unrelated patients and tapered on days +30 to +80, based on the presence or absence of severe GVHD, infectious diseases and liver or renal disorders. CD25 monoclonal antibody (basiliximab; Novartis Pharma Schweiz, Postfach, Switzerland) was given at 10 mg/m2 per day intravenously once 2 h before the infusion of cells collected in HLA-antigen-mismatched, unrelated transplantation patients. Acute and chronic GVHD was diagnosed and graded according to the Fred Hutchinson Cancer Research Center (FHCRC) criteria19, 20. GVHD was treated with 1 to 2 mg/kg per day of methelprednisolone and resumption of full-dose CSA administration. Second-line immunosuppressive therapy, such as tacrolimus (FK506), MMF, and CD25 monoclonal antibody or MTX, was given for steroid refractory aGVHD, and FK506 was administered in CSA-refractory extensive cGVHD.
Infection prevention and support care
All patients were cared for in private rooms with positive pressure and a high-efficiency particle filtration air system. They received prophylactic antibiotics when the ANC was less than 1×109/L. Fluconazole was given orally from day -5 until engraftment, trimethoprim sulfamethoxazole was administered orally for prophylaxis of Pneumocysis carinii infection, acyclovir was given orally from days -10 to +30 and ganciclovir was routinely used intravenously from days -10 to -2. Patients' levels of serum CMV-DNA, C-reaction protein and beta-lactam (GM index) detection were monitored weekly. Blood products were irradiated to 2500 cGy, and CMV-seronegative recipients received leucodepleted and irradiated blood products. Alprostadil (20 μg twice a day) was administered intravenously on days +1 to +28, and ursofalk (Losan Pharma GmbH, Neuenburg, Germany, 250 mg twice a day) was used orally beginning on day +1 to prevent liver veno-occlusive disease (VOD) and GVHD. Uroepithelial protection was achieved with intravenous hydration, and mesna was used during CY administration. All patients received phenobarbital at a dose of 2 mg/kg twice a day from day -1 until the end of the conditioning regimen to prevent seizures. Total parenteral nutrition was provided as needed. Additional agents were added on the basis of patients' clinical status and the results of pathogen reports when fever, severe mucositis, or new infections occurred during prophylaxis. Day 0 was the day of donor cell infusion.
Grading of toxicity
Regimen-related toxicity (RRT) was graded according to the criteria published by Bearman et al19. RRT was graded when organ damage was not identified as the result of infection, GVHD or drug therapy and was assumed to be caused by the preparative regimen.
Chimerism analyses
Chimerism was typically evaluated on recipient bone marrow cells on days +30, +180, and +360 using PCR-based analyses of polymorphic minisatellite or microsatellite regions. HLA typing was performed for patients after mismatched transplantation18.
Study definitions
The modified BUCY2 conditioning regimen administered in this study was defined according to the effective regimen used by Lu et al18 in China. Criteria for a complete response included normal cytogenetics, the absence of circulating blasts, less than 5% marrow blasts, and a platelet count of 100×109/L or higher. Standard morphologic criteria and immunophenotype analysis by flow cytometry were used to diagnose recurrent disease. Response was documented as the best response occurring after day 30 following allo-SCT. A molecular response measured by the quantitative polymerase chain reaction analysis for BCR-ABL rearrangement was obtained when necessary. Hematologic recovery was defined on the date that a patient had an absolute neutrophil count of 0.5×109/L or higher for 3 consecutive days. Platelet recovery was defined as occurring on the first of 7 consecutive days with a platelet count of 20×109/L or higher without transfusion support. Failure to engraft by day +30 was considered primary engraftment failure1.
Early regimen-related toxicity was defined as that occurring within 100 days after transplantation20. Overall survival (OS) was defined as the time from the date of transplant until death from any cause or until the last follow-up. Leukemia-free survival (LFS) was defined as the time from the date of transplant until disease relapse, progression or death or until the last follow-up in CR. Treatment-related mortality (TRM) was defined as any cause of death other than the underlying disease.
Statistical analysis
Patient-, disease-, and transplant-related variables for patients receiving IDA- and VP16-intensified conditioning regimens were compared using chi-square statistics or Fisher's exact test for category variables and the Wilcoxon Rank Sums (two-sided) for continuous variables. The actuarial probabilities of survival, relapse, TRM, OS, and LFS were estimated using the method of Kaplan and Meier. Comparison of survival curves for the two treatment groups was performed by the log-rank test. The following features were studied: type of acute leukemia (AML versus ALL), patients' sex (male versus female), patients' age (< versus > median age), disease phase at time of transplant (CR versus NR), CNS-leukemia (yes versus no), HLA type (HLA-identical sibling, mismatched related, matched unrelated or mismatched unrelated), acute GVHD (0, 1–2, or 3–4), chronic GVHD (no versus yes), ANC and platelet engraftment (< versus > median time), CMV infection (no versus yes), Aspergillus pneumonia (no versus yes), seizure (no versus yes) and conditioning regimen (IDA versus VP16). A P value <0.05 was considered statistically significant. A Cox proportional hazards model was used in multivariate analyses for outcomes. The final multivariate models were built using a forward stepwise model selection approach. Each model contained the main effect for IDA-intensified regimen versus VP16-intensified regimen groups, since this was the main interest of the study. The potential interaction between the main effect and all significant covariates was tested. No interactions were detected. Adjusted probabilities of LFS and OS were calculated using the multivariate models. Ninety-five percent confidence intervals (CI) were reported for the main summary statistics6, 18. Results were analyzed as of November 30, 2008.
Results
Pretransplantation patient and disease characteristics
Forty-eight patients (n=28 and 20 for the IDA and VP16 groups, respectively) with high-risk acute leukemia were treated between January 2005 and June 2008. The patients in both groups were matched for the possible confounding variables of age, gender, diagnosis, phase of underlying diseases (AML or ALL, high WBC count, central nervous system leukemia (CNS-leukemia), secondary to MDS, and remission status before transplant), performance status, ABO match and donor HLA type. A comparison of the IDA group and VP16 group with regard to these variables showed no differences between the groups (Table 1).
Hematopoietic reconstitution
Analysis of chimerism indicated that all patients achieved full donor chimerism by day 30 after allo-PBSCT. All patients engrafted to an ANC exceeding 0.5×109/L, with a median time to neutrophil engraftment of 12 days (range, 9–19 days) in the IDA group versus 16 days (range, 11–26 days) in the VP16 group (T=671.500, P<0.001). Patients achieved platelet engraftment in the IDA and the VP16 groups at 13 days (range, 11–25 days) versus 18 days (range, 10–29 days), respectively (T=647.000 and P=0.002). The IDA group required significantly less time to achieve hematopoietic reconstitution.
Early regimen-related toxicity
All patients received the conditioning regimen as prescribed, and the treatment was in no case interrupted because of side effects. No patient developed grade 3 (life-threatening) or 4 (fatal) early regimen-related toxicity. Up to and including day +28 after transplantation, no patient died in either of the two groups. Between days +29 and +100, three patients in the VP16 group died after a relapse; on days +35 and +43, Aspergillus pneumonia was diagnosed in two of the three patients with relapse.
Grades 3 and 4 organ toxicities were evaluated during the 40 days after allo-SCT for the IDA group versus the VP16 group, respectively, as follows: hepatic, 7% versus 8%; hepatic veno-occlusive, 0% versus 5%. No early regimen-related neurologic, cardiovascular or renal toxicities occurred, and no death resulted from lethal organ toxicities in the two groups during the 40 days after allo-SCT. One patient from the IDA group had a seizure on day +68 and had no eyesight from day +130 forward. Four and six patients experienced grade 3 nausea in the IDA and VP16 groups, respectively, and three and four patients had grade 3 vomiting in these same groups. Mucositis was observed in 23 (82%) and 9 (45%) patients from the IDA and VP16 groups, respectively. Thus, the IDA-intensified regimen was more likely to cause mucositis than the VP16-intensified regimen (P<0.01). There were no grade 4 toxic events of the gastrointestinal tract for any patients. Aside from the incidents of pneumonia already described, there were no adverse pulmonary events.
GVHD incidence and severity
As shown in Tables 2 and 3, no significant difference (P<0.05) in terms of acute or chronic GVHD incidence or severity was observed between the two groups. With the exception of one patient with grade 4 aGVHD who died of Aspergillus pneumonia on day +164, all patients in the IDA group with aGVHD were successfully controlled with intravenous corticosteroids or a second line of immunosuppressive drugs (tacrolimus, MMF, MTX, or CD25 monoclonal antibody). In the VP16 group, three (15%) patients had grade 2 to 4 aGVHD. One of these patients progressed into grade 4 aGVHD, with severe bloody diarrhea and a skin rash, on day +37. After a combination treatment of tacrolimus and the CD25 monoclonal antibody, the patient experienced a relapse on day +67 and died on day +102.
Infectious complications
The 100-day cumulative incidences of cytomegalovirus (CMV) antigenemia, Aspergillus pneumonia, and bacterial infection for the IDA versus VP16 groups were 25% (CI, 19%–31%) versus 30% (CI, 23%–37%) (P=0.70), 71% (CI, 66%–17%) versus 40% (CI, 34%–47%) (P=0.03) and 39% (CI, 32%–46%) versus 40% (CI, 33%–46%) (P=0.09), respectively. No hemorrhagic cystitis (HC) was noted in either group within 100 days. One patient from the IDA group died of pneumonia complicated with diffuse alveolar hemorrhage (day +70). These results suggested that patients conditioned with the IDA-intensified regimen had a higher risk of Aspergillus pneumonia infection.
Outcome
The median follow-up periods in the IDA and VP16 groups were 298 days (range, 53–845 days) and 395 days (range, 70–790 days), respectively. Twenty-one patients from the IDA group were alive at a median of 499 days (range, 227–790 days), and 11 from the VP-16 group were alive at a median of 377 days (range, 225–845 days); the other 16 patients (7 and 9 from the IDA and VP16 groups, respectively) had died. Eight patients (28%) in the IDA group and 10 (50%) in the VP16 group relapsed (IDA versus VP16, Χ2=2.280, P=0.130). One patient in the VP16 group relapsed on day +110 and then received combination chemotherapy consisting of fludarabine (Flud) and cytarabine (Ara-C) concurrent with a transfusion of donor hematopoietic stem cells21; this patient achieved CR, which was still in effect at the time of this writing. One patient in the IDA group relapsed with extramedullary leukemia on day +310 and was still alive at the end of the study (day +330). All of the other 16 patients experiencing a relapse died, and two of them died of transplant-related causes. Although two patients in the IDA group and no patients in the VP-16 group died of transplant-related toxicities, no statistically significant difference in the transplant-related mortality (TRM) was found (P=0.500). These results show that the two regimens did not differ in a statistically significant manner with regard to the relapse rate or TRM.
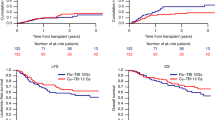
Adjusted for other prognostic variables identified in the multivariate analysis, the 2-year probabilities of LFS were 72% (95 CI, 64%–80%) and 51% (95 CI, 42%–57%) after transplantations conditioned with the IDA- and VP16-intensified regimens, respectively (P=0.04, Figure 1A). Meanwhile, the adjusted 2-year OS probabilities were 74% (95 CI, 67%–82%) and 53% (95 CI, 43%–60%) for patients of the two groups, respectively (P=0.04) (Figure 1B). As assessed by the LFS and OS, therefore, these results demonstrate that patients benefited more from the IDA-intensified conditioning regimen before allo-SCT than from the VP16-intensified conditioning regimen before allo-SCT. After adjusting for the type of acute leukemia, patients' sex, age, disease phase at the time of transplant, CNS-leukemia and donor HLA type, the regimen used significantly affected the OS (P=0.04) but had no impact on the relapse risk or TRM (P>0.05).
LFS and OS of the two treatment group show significant differences. Patients conditioned with IDA-intensified regimens demonstrated better leukemia-free survival (A, P=0.04) and overall survival (B, P=0.04) than patients given VP16-intensified regimens. LFS, leukemia-free survival; OS, overall survival; IDA, idarubicin; VP16, etoposide.
Discussion
Allogeneic graft-versus-tumor effects are powerful and can lead to cures of incurable hematological malignancies. They work best in patients with relatively low tumor burdens and slow-growing tumors, and they are least effective in patients who have bulky tumors with relatively fast proliferation rates (eg, acute leukemia patients in relapse)12. However, myeloablative regimens should in principle be more appropriate in patients with high-risk acute leukemia6, 12, 20. Few studies published consider the comparison of different intensified conditioning regimens associated with LFS and OS for high-risk acute leukemia. The present study is the first to compare IDA and VP16 in intensified conditioning regimens for allo-SCT. In this study, we retrospectively analyzed the outcome of 48 patients with high-risk acute leukemia who underwent allo-SCT following myeloablative conditioning regimens intensified with IDA or VP16. Our data demonstrate that patients with high-risk acute leukemia may benefit more from allo-SCT with an IDA-intensified conditioning regimen than with a VP16-intensified conditioning regimen.
The choice of IDA in combination with BUCY2 was made mainly on the basis of 1) IDA's major anti-leukemic activity against the multidrug-resistant cell compartment and 2) the lower cardiac toxicity of IDA compared to other anthracyclines22. In our study, mucositis was comparable in terms of incidence and severity to that observed by Mengarelli et al23 in recipients of T cell-depleted marrow conditioned with a TBI-based regimen intensified with IDA. IDA in our study was given at 15 mg/m2 per day intravenously on days -12 to -10; Mengarelli et al23 reported a regimen consisting of IDA at a dose of 21 mg/m2 per day for two days. Similar to the findings observed in the study of Mengarelli et al, no patients developed life-threatening or fatal early regimen-related toxicity in our study23. This allowed us to explore the possibility and feasibility of an IDA-intensified regimen for high-risk acute leukemia treatment before allo-SCT. Henwood et al24 reviewed the anti-tumor activities of VP16 with low non-hematological toxicities. Kröger et al25 evaluated the efficacy and toxicity of two different VP-16 dosages (30 or 45 mg/kg) in combination with BUCY as a conditioning therapy before allo-SCT in acute myeloid leukemia (AML). In all, 90 patients with AML received either 30 mg/kg (n=60) or 45 mg/kg (n=30) of VP16. This study concluded that VP16 (at either 30 mg/kg or 45 mg/kg) in combination with BUCY serves as a highly active regimen for the treatment of AML patients undergoing bone marrow transplantation and produces a low relapse rate. Further, conditioning with 30 mg/kg rather than 45 mg/kg of VP16 resulted in less toxicity and a better OS owing to the lower TRM. In the present study, a VP16 dosage of 25 mg/kg was chosen to induce less toxicity in refractory acute leukemia patients with previously existing, chemotherapy-related toxicities. The comparison of grade 3 to 4 organ toxicities during the 40 days after allo-SCT revealed that no significant differences in terms of hepatic and hepatic veno-occlusive toxicities were observed; further, no early regimen-related neurological, cardiovascular, or renal toxicities occurred, and no death resulted from lethal organ toxicities in either group. However, mucositis was observed in 23 (82%) and 9 (45%) patients from the IDA and VP16 groups, respectively. There was therefore a greater risk for mucositis in patients treated with the IDA-intensified regimen than in those with the VP16-intensified regimen (P<0.01). The 100-day cumulative incidence of Aspergillus pneumonia was 71% for the IDA group; this value was significantly higher than that for the VP16 group (P<0.01). Interestingly, the high incidence of Aspergillus pneumonia paralleled that of mucositis in the IDA group and may have been due in part to mucosal barrier injury. After treatment with antifungal drugs, three patients from the VP16 group died after relapse between days +29 and +100. Two of these patients were diagnosed with Aspergillus pneumonia on days +35 and +43; however, Aspergillus infection was not correlated with early TRM in either group.
No significant difference between the two groups with regard to the incidence or severity of acute or chronic GVHD incidence or the relapse rate was observed. The low incidences of aGVHD and cGVHD likely played a significant role in the tolerability of the transplant conditioning with our intensified regimens. Because of the small number of patients, we were not able to establish whether the difference in the risk of relapse between AML and ALL patients was due to a major antileukemic activity of the regimen or instead to a major GVL effect against myeloid compared to lymphoid blasts. Our results indicate that the different outcomes of these two groups were due to neither the underlying disease nor incidence of GVHD, however, because there were no statistically significant differences between the two groups with regard to patient characteristics before the conditioning regimen treatment.
To our surprise, patients treated with the IDA-intensified regimen achieved a significantly better LFS and OS (P=0.04). This finding suggests that patients with high-risk acute leukemia can benefit more in terms of survival from a conditioning regimen consisting of IDA than VP16. Multivariate analysis of our results from patients with high-risk acute leukemia showed that the alternative conditioning regimen was the strongest prognostic factor and that disease phase before all-SCT was the second strongest prognostic factor for both OS and LFS. The outcome results for the IDA group were not entirely consistent with those from a previous study. In a large retrospective study examining factors that affect the outcome of allogeneic BMT for adults with refractory or relapsed acute leukemia, Grigg et al26 confirmed the very poor prognosis of 383 patients with high-risk features. These authors reported OSs of 30%, 23%, 18%, and 14% at 1, 2, 5, and 10 years, respectively. Mengarelli et al23 reported that the IDA-BUCY2 regimen allowed a 2-year LFS probability of 53% without an unacceptable increase of RRT; this regimen seems particularly promising in advanced AML patients. The patients with the IDA-intensified regimen in the present study achieved better LFS and OS but similar RRT outcomes in comparison to those from published studies.
The current study had some limitations. Although patient characteristics were not significantly different between the two treatment groups, the underlying diseases were heterogeneous. Selection of a conditioning regimen was not randomized; the IDA and VP16 regimens were avoided if the patient had previously demonstrated resistance. This bias may have influenced our results. In particular, the patient group size in the present study inevitably influenced our results. A few conclusions from this study may be considered in future studies focused on the selection of a conditioning regimen for high-risk acute leukemia patients. The IDA-intensified conditioning regimen at the doses used in this study is significantly superior to the VP16-intensified conditioning regimen for high-risk acute leukemia patients. It permits better DFS and OS, despite the higher incidence of Aspergillus pneumonia and mucositis. However, the anti-leukemic and survival properties of the two intensified regimens should be confirmed in a larger sample of patients with a longer follow-up period.
Author contribution
Yong YOU and Ping ZOU designed the research; Yong YOU, Zhi-chao CHEN, Linghui XIA, and Ping ZOU performed the research; Qiu-bai LI and Lei LI analyzed the data; Qiu-bai LI wrote the paper.
References
Copelan EA . Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354: 1813–26.
Shimoni A, Hardan I, Shem-Tov N, Rand A, Herscovici C, Yerushalmi R, et al. Comparison between two fludarabine-based reduced-intensity conditioning regimens before allogeneic hematopoietic stem-cell transplantation: fludarabine/melphalan is associated with higher incidence of acute graft-versus-host disease and non-relapse mortality and lower incidence of relapse than fludarabine/busulfan. Leukemia 2007; 2: 2109–16.
Thomas ED . Marrow transplantation for malignant disease (Karnofsky Memorial Lecture). J Clin Oncol 1983; 1: 517–31.
Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P, et al. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood 2008; 112: 3574–81.
Laport GG, Alvarnas JC, Palmer JM, Snyder DS, Slovak ML, Cherry AM, et al. Long-term remission of Philadelphia chromosome-positive acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation from matched sibling donors: a 20-year experience with the fractionated total body irradiation-etoposide regimen. Blood 2008; 112: 903–9.
Mengarelli A, Iori A, Guglielmi C, Romano A, Cerretti R, Torromeo C, et al. Standard versus alternative myeloablative conditioning regimens in allogeneic hematopoietic stem cell transplantation for high-risk acute leukemia. Haematologica 2002; 87: 52–8.
Anderson JE, Gooley TA, Schoch G, Anasetti C, Bensinger WI, Clift RA, et al. Stem cell transplantation for secondary acute myeloid leukemia: evaluation of transplantation as initial therapy or following induction chemotherapy. Blood 1997; 89: 2578–5.
Marks R, Potthoff K, Hahn J, Ihorst G, Bertz H, Spyridonidis A, et al. Reduced-toxicity conditioning with fludarabine, BCNU, and melphalan in allogeneic hematopoietic cell transplantation: particular activity against advanced hematologic malignancies. Blood 2008; 112: 415–25.
Huang XJ . Advance in hematopoietic stem cells transplantation for leukemia. Chin Med J (Engl) 2008; 121: 1763–5.
Kolb HJ, Schmid C, Barrett AJ, Schendel DJ . Graft-versus-leukemia reactions in allogeneic chimeras. Blood 2004; 103: 767–76.
Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995; 86: 2041–50.
Kahl C, Storer BE, Sandmaier BM, Mielcarek M, Maris MB, Blume KG, et al. Relapse risk in patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood 2007; 110: 2744–8.
Vaughan WP, Dennison JD, Reed EC, Klassen L, McGuire TR, Sanger WG, et al. Improved results of allogeneic bone marrow transplantation for advanced hematologic malignancy using busulfan, cyclophosphamide and etoposide as cytoreductive and immunosuppressive therapy. Bone Marrow Transplant 1991; 8: 489–95.
Hirabayashi N, Goto S, Ishii M, Yuge M, Mitsuma A, Noda N . Busulfan, cyclophosphamide and total body irradiation as conditioning for allogeneic bone marrow transplantation for acute and chronic myeloid leukemia. Bone Marrow Transplant 1998; 21: 1079–83.
Brown RA, Wolff SN, Fay JW, Pineiro L, Collins RH Jr, Lynch JP, et al. High-dose etoposide, cyclophosphamide and total body irradiation with allogeneic bone marrow transplantation for resistant acute myeloid leukemia: a study by the North American Marrow Transplant Group. Leuk Lymphoma 1996; 22: 271–7.
Blume KG, Kopecky KJ, Henslee-Downey JP, Forman SJ, Stiff PJ, LeMaistre CF, et al. A prospective randomized comparison of total body irradiation-etoposide versus busulfan-cyclophosphamide as preparatory regimens for bone marrow transplantation in patients with leukemia who were not in first remission: a Southwest Oncology Group Study. Blood 1993; 81: 2187–93.
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000; 96: 4075–83.
Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood 2006; 107: 3065–73.
Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol 1988; 6: 1562–8.
Tauro S, Craddock C, Peggs K, Begum G, Mahendra P, Cook G, et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol 2005; 23: 9387–93.
You Y, Li QB, Chen ZC, Li WM, Xia LH, Zhou H, et al. Fludarabine and cytarabine combined chemotherapy followed by transfusion of donor blood stem cells for treating relapse of acute leukaemia after allogeneic haematopoietic stem cell transplantation. Chin Med J (Engl) 2008; 121: 1770–4.
Berman E, McBride M . Comparative cellular pharmacology of daunorubicin and idarubicin in human multidrug resistant leukemia cells. Blood 1992; 79: 3267–76.
Mengarelli A, Iori AP, Guglielmi C, Perrone MP, Gozzer M, Girmenia C, et al. Idarubicin intensified BUCY2 regimen in allogeneic unmanipulated transplant for high-risk hematological malignancies. Leukemia 2000; 14: 2052–8.
Henwood JM, Brogden RN . Etoposide . A review of its pharmacodynamic and pharmacokinetic propertyes, and therapeutic potential in combination chemotherapy of cancer. Drugs 1990; 39: 438–90.
Kröger N, Zabelina T, Sonnenberg S, Krüger W, Renges H, Stute N, et al. Dose-dependent effect of etoposide in combination with busulfan plus cyclophosphamide as conditioning for stem cell transplantation in patients with acute myeloid leukemia. Bone Marrow Transplantation 2000; 26: 711–6.
Grigg AP, Szer J, Beresford J, Dodds A, Bradstock K, Durrant S, et al. Factors affecting the outcome of allogeneic bone marrow transplantation for adult patients with refractory or relapsed acute leukemia. Br J Haematol 1999; 107: 409–18.
Acknowledgements
The present work was partly supported by a grant from the Key Project Fund of the Hubei Bureau of Health (No JX2A10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Qb., Li, L., You, Y. et al. A comparative study of outcomes of idarubicin- and etoposide-intensified conditioning regimens for allogeneic peripheral blood stem cell transplantation in patients with high-risk acute leukemia. Acta Pharmacol Sin 30, 1471–1478 (2009). https://doi.org/10.1038/aps.2009.132
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2009.132