Abstract
Aim:
Since the distal part of the intestine is targeted by a wide range of pathogens, the motility of the recto-anal region has been the object of many experimental and clinical observations. In this study, we investigated descending motor responses in the anal canal as a measure of the activation of autonomic reflex pathways underlying evacuatory recto-anal activity.
Methods:
The partitioned organ bath method was used to register motor responses of the anal canal as induced by balloon distension of the rectum in isolated rat recto-anal preparations.
Results:
Distension-induced descending responses of the anal canal comprised contractions (with distension at a distance of 15 mm), initial contractions and secondary relaxations (at 10 mm) and short contractions followed by deep relaxations (at 3−5 mm). Decreasing the distance between the distension stimulus and the anal canal resulted in a decreased contraction response and increased relaxation. Tetrodotoxin (0.1 μmol/L) inhibited these responses. Atropine (0.3 μmol/L) decreased contraction and did not change the relaxation response. NG-nitro-L-arginine (0.5 mmol/L) enhanced contraction in both the absence and presence of atropine. L-arginine (0.5 mmol/L) inhibited contraction and extended relaxation in atropine-pretreated preparations. The actions of NG-nitro-L-arginine and L-arginine were more pronounced in the aboral direction. ChAT-positive nerve fibers were observed in myenteric ganglia of the rectum and the anal canal. The density of NADPH-diaphorase-positive neurons was higher in the anal canal region.
Conclusion:
Our results suggest that locality-dependent activation of the descending reflex neuromuscular communications underlie evacuatory activity in the recto-anal region. This activation response involves long excitatory cholinergic and non-cholinergic pathways along the rectum and short inhibitory nitrergic pathways located predominantly in the anal canal region.
Similar content being viewed by others
Introduction
Because of the clinical and social impact of large intestine-related diseases, the motor activity of the recto-anal area of the large bowel is of increased interest. The recto-anal evacuatory mechanism is a complex process involving myogenic properties and innervations in the rectum and internal and external anal sphincters. The recto-anal region is regulated by a dual nerve supply, somatic and autonomic1. Mechanographic and electrophysiological observations have demonstrated that the motor activity of the mammalian large intestine can occur in isolated preparations, thus indicating that the reflex pathways underlying intestinal motility are contained within the gut wall2, 3, 4, 5.
In the recto-anal region, colorectal stimulatory6, recto-colonic inhibitory7, 8 and colo-anal9, ano-rectal10, and recto-anal excitatory11, 12 reflex pathways have been demonstrated. It is believed that the physiological significance of recto-anal evacuatory activity is mainly attributed to the autonomic recto-anal inhibitory reflex underlying the functional nature of rectal discrimination. The recto-anal inhibitory reflex consists of distension-evoked rectal reflex contraction and synchronous internal anal sphincter reflex relaxation, highlighting the importance of the propulsive capacity of the internal anal sphincter13, 14, 15, 16, 17.
The contribution of recto-anal neurotransmission to the contractile and/or relaxant activity of the internal and external anal sphincters requires further investigation. According to Bharucha et al1, both anal sphincters are responsible for the maintenance of neurogenic recto-anal motility. We failed to find experimental data demonstrating coordination between rectal autonomic nerve pathways and the motor responses of the anal canal in the presence of preserved anatomical and functional integrity of the internal and external anal sphincters.
In the present study, we have reexamined reflex evacuatory activity in the recto-anal region using a rat recto-anal preparation as an experimental model. The properties of the defecation reflex in the rat have scarcely been investigated18. In particular, we were interested in evaluating the descending motor responses of the anal canal in response to balloon inflation-induced local distension of the rectal wall. This response was examined at different distances from the anal canal to evaluate the topography of descending motor reflex pathways that control the evacuatory motility of the anal sphincters. The partitioned organ bath method was used to register balloon inflation-induced motor responses in the anal canal. To evaluate the contributions of cholinergic and nitrergic neurotransmissions to descending reflex pathways, the motor responses of the anal canal were pharmacologically analyzed using cholinergic- and nitrergic-related drugs. Morphological techniques, which allowed us to evaluate the presence and distribution of choline acetyltransferase (ChAT) and nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-diaphorase), were used to identify acetylcholine- and nitric oxide-containing nerve structures.
Materials and methods
Experiments were carried out in the Laboratory of Peripheral Synapses in the Institute of Neurobiology of the Bulgarian Academy of Sciences and were approved by the Animal Care and Use Ethics Committee of the Institute of Neurobiology.
Animals
Eighteen male rats that weighed between 250−280 g were euthanized. The animals were starved overnight, stunned by a blow to the neck and decapitated. The abdominal cavity was opened and the pubic symphysis was cut away exposing the large intestine. The perianal skin was excised and the anal canal, including the distal part of the large intestine, was removed and placed in modified Krebs solution at room temperature. Fecal pellets were removed by gently flushing the lumen with Krebs solution which was applied with a syringe to the oral end of the preparation. The extrinsic blood vessels and nerves along the mesenteric border were then carefully trimmed away. A segment consisting of the rectum and the anal canal with intact nerve plexuses-smooth muscle layers was isolated (recto-anal preparation, 23−25 mm in length)19.
Protocol design
The recto-anal preparation was mounted in a partitioned organ bath and was allowed to equilibrate for 45 min before initiating the experiment.
Balloon inflation-induced descending motor responses of the anal canal were registered before and during drug treatment. The drugs were added into the compartment of the partitioned bath which contained the isolated recto-anal segment preparation. The drugs were administered in volumes not exceeding 0.5%−1% of the compartment's volume. The time course and concentration for drug treatment was as follows: tetrodotoxin, 0.1 μmol/L, 10 min; atropine, 0.3 μmol/L, 15 min; NG-nitro-L-arginine, 0.5 mmol/L, 15 min; and L-arginine, 0.5 mmol/L, 15 min. When the drugs were added consecutively (atropine plus NG-nitro-L-arginine or atropine plus L-arginine) the time course was 30 min12, 19.
Partitioned organ bath method
A modified partition organ bath method for studying reflex motor responses in isolated intestinal segments was used20. The flat organ bath was divided into two compartments by a plastic partition that contained a slit filled with a paraffin “diaphragm,” thus representing an oral and an anal compartment. Each compartment was supplied by a self-dependent, continuous perfusion system with gassed Krebs solution. The recto-anal segment was gently threaded through a 2-mm- diameter hole in the paraffin diaphragm. The rectum (20−22 mm in length) was placed in the oral compartment while the anal canal (3−5 mm in length) was situated in the anal compartment of the bath. The proximal end of the rectum was tied with silk thread to the proximal side of the oral compartment. The motor activity of the anal canal was measured at two opposite sites of the ring circumference, one of which was secured to a plastic rod and the other which was connected to a strain gauge under an initial tension equivalent to 10 mN. Thus, interference by movement of the rectal part of the segment preparation was minimized at the securing point. As such, anal canal responses to balloon-induced distension of the rectum did not result in mechanical artifacts. Inert silicone grease was then applied around the gut circumference attached to the paraffin diaphragm to prevent contact between the solutions in the two different compartments (Figure 1).
Schematic drawing of the set up for studying balloon inflation-induced descending reflex motor activity of the anal canal in an isolated rat recto-anal segment placed in a partitioned organ bath with compartments for the rectum and anal canal. Designations: registration of motor activity of the anal canal (M); positions of balloon inflation along the rectum at distances of 3−5, 10, or 15 mm from the anal canal.
Balloon-induced distension
Balloon-induced distension of the rectum was performed to activate autonomic nervous pathways within the rectal wall. Distension was performed using a polyethylene, balloon-tipped, fine plastic tubule connected to a micro-syringe of 1.0 mL volume. The size of the balloon, which was inflated with Krebs solution (stepwise volume-controlled inflation with 0.04−0.40 mL solution at 36.5 oC), imitated the size of rat excrement (2.5−3.0 mm in diameter). In order to identify any possible topographical parameters of the recto-anal reflex, we measured the length of the reflex pathways underlying evacuatory motor activity. For this, the empty balloon was pushed through the lumen of the proximal part of the rectum in the recto-anal preparation. After a period of equilibration, the empty balloon was gently moved in the aboral direction and inflated and deflated slowly (30 s) at different positions along the entire length of the rectum at distances of 3−5, 10 or 15 mm away from the anal canal.
Equipment
Strain gauges (Microtechna, Prague, Czech Republic), two-channel stimulators and amplifiers (Experimetria, Budapest, Hungary) and two-channel TZ 4620 recorders (Laboratorni pristroje, Prague, Czech Republic) were used for recording of motor responses.
Statistical analyses
The spontaneous muscle tone of the anal canal was used as baseline when measuring the magnitude of the force of motor responses in mN. Data are presented as the mean±SEM. Statistical significance was assessed using the Student's t-test for paired and unpaired samples. Value of P<0.05 were considered significant. Statistical calculations were obtained by using software21.
Immunohistochemical and histochemical analyses
The expression and distribution of acetylcholine and nitric oxide in neuronal structures of the recto-anal region were examined by ChAT-immunohistochemistry and NADPH-diaphorase histochemistry. Four male rats were euthanized. Upon undergoing anesthesia, the animals were perfused with 4% paraformaldehyde in 0.1 mol/L phosphate buffer. The recto-anal region was removed and fixed again in the same fixative solution for 24 h. Pairs of tissue sections of 30 μm thickness were cut on a freezing microtome. Half of the sections were processed for ChAT immunohistochemistry by the ABC (avidin-biotin-horseradish peroxidase) method22 and the other half were processed for NADPH-diaphorase histochemistry. For ChAT-immunohistochemistry, the sections were washed with phosphate buffered saline (PBS)/0.3% Triton X-100 and then incubated in 10% normal rabbit serum for 60 min. After a brief rinse with PBS, the sections were incubated for 24 h in a solution containing polyclonal goat anti-ChAT, diluted 1:200, and later incubated in a solution of anti-goat IgG-biotin, diluted 1:250, for 90 min. Afterwards, the sections were incubated for 90 min in ABC complex diluted 1:100. The reaction was visualized by incubating with 3,3'-DAB/H2O2. The intensity of the reaction was increased with 0.05% nickel ammonium sulfate. Control sections were incubated in the absence of the primary antibody and the results were negative. A modified method for NADPH analysis by Scherer-Singler et al23 was used for the histochemical reaction. By visual inspection, the staining intensity of NADPH-d-positive nerve structures was estimated as low (+), moderate (++) or high (+++).
Morphological equipment
A Reichert Jung freezing microtome (Austria), a light microscope (Jenaval, Germany) and a digital photocamera (Nikon, Japan) were used.
Solutions and drugs used in motor reflex studies
Modified Krebs solution consisted of 120 mmol/L NaCl, 5.9 mmol/L KCl, 15.4 mmol/L NaHCO3, 1.2 mmol/L NaH2PO4, 1.2 mmol/L MgCl2, 2.5 mmol/L CaCl2, and 11.5 mmol/L glucose. The solution was continuously aerated in 95% O2 and 5% CO2 (pH 7.2) at 36.5 oC.
The drugs used in our studies included tetrodotoxin (TTX, Sankyo, Zurich, Switzerland), atropine sulfate (Merck, Darmstadt, Germany), NG-nitro-L-arginine (L-NNA), and L-arginine (Sigma Chemicals, St Louis, MO, USA). Drugs were dissolved in distilled water and diluted to their final concentration in Krebs solution prior to use. TTX stock solution was stored at -18 oC.
Solutions and drugs used for morphological studies
0.05 mol/L PBS pH 7.3, 0.1 mol/L phosphate buffer (PB) pH 7.3, 0.05 mol/L Tris-HCl buffer pH 7.4 and 7.56, Triton X-100, hydrogen peroxide (Fluka AG, Buch, Switzerland), paraformaldehyde, Entellan mounting media (Merck, Darmstadt, Germany), normal rabbit serum, reduced β-nicotinamide adenine dinucleotide phosphate (β-NADPH), nitroblue tetrazolium chloride (NBT), 3,3'-diaminobenzidine tetrahydrochloride (3,3'-DAB) (Sigma Chemicals, St Louis, MO, USA), polyclonal goat anti-choline acetyltransferase (anti-ChAT) antibody, rabbit IgG-biotin antibody (Chemicon Inc, Billerica, MA, USA) and avidin-biotin complex (Vectastain ABC kit, Vector Laboratories Inc, Burlingame, USA).
Results
Balloon distension-induced descending motor responses
Distension of the rectal wall by inflation of the balloon, positioned at a distance of 3−5, 10, or 15 mm away from the anal canal, induced differentially patterned descending motor responses in the anal canal.
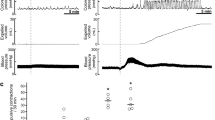
Fast contractions, which declined during balloon-induced distention of the rectum, were observed when the balloon was inflated in the proximal part of the rectum, 15 mm away from the anal canal (Figure 2A). When the balloon was positioned in the middle part of the rectum, 10 mm away from the anal canal, the descending response consisted of an initial short contraction followed by relaxation. The latter contraction was smaller in amplitude compared to the contraction caused by inflation of the balloon in the proximal part of the rectum, 15 mm away from the anal canal (2.41±0.26 mN and 4.04±0.36 mN, n=14, P<0.01, respectively). The relaxation response (1.34±0.18 mN, n=14) faded during balloon-induced distention of the rectal wall (Figure 2B). Balloon-induced distension of the distal part of the rectum, at 3–5 mm away from the anal canal, again resulted in a dual motor response in the anal canal–a short low-amplitude contraction (1.62±0.22 mN) followed by long-lasting, deep relaxation (2.88±0.36 mN) (Figure 2C). The contraction and relaxation responses induced by the latter stimulus significantly differed from those of the response due to rectal distension 10 mm away from the anal canal (n=14, P<0.01).
Typical mechanographic records showing descending reflex motor responses of the anal canal (Sie) in an isolated rat recto-anal segment during balloon inflation-induced distension of the rectum 15 mm away from the anal canal for 30 s (A); 10 mm away from the anal canal (B); and 3−5 mm away from the anal canal (C).
Drug-induced modulation of balloon distension-induced descending motor responses
Atropine, applied at a concentration of 0.3 μmol/L in the nutrient solution of the anal compartment of the bath, converted the descending contraction of the anal canal into a contraction followed by relaxation when balloon-induced distension of the rectum was applied 15 mm away from the anal canal. Contraction after treatment with atropine was less pronounced than that before atropine treatment. L-NNA (0.5 mmol/L) significantly increased the descending contraction both in the absence and presence of atropine. The response of the anal canal to the addition of L-arginine (0.5 mmol/L) in the presence of atropine resembled the response pattern obtained after application of atropine alone; the contraction was noticeably reduced and relaxation was noticeably increased (Figure 3).
Descending reflex motor responses of the anal canal (Sie) in an isolated rat recto-anal segment during balloon inflation-induced distension of the rectum for 30 s, 15 mm away from the anal canal; 10 mm away from the anal canal; and 3−5 mm away from the anal canal. Designations for descending reflex motor responses: controls (C) and in the presence of atropine (Atr, 0.3 μmol/L), NG-nitro-L-arginine (L-NNA, 0.5 mmol/L), atropine plus NG-nitro-L-arginine (Atr+L-NNA) and atropine plus L-arginine (0.5 mmol/L) (Atr+L-Arg). Values represent the mean±SEM of at least 8 experiments. Symbols indicate: significant differences at P ≤0.05, t-test (t≥2.31 for 8 experiments) - (b) drug treatment vs controls and (♦) opposite effects vs controls.
The initial contraction of the descending response in the anal canal, which was observed when balloon-induced distension was applied 3−5 or 10 mm away from the anal canal, decreased with atropine treatment, while the relaxation response was unchanged. In both cases, L-NNA increased contraction and significantly decreased relaxation before and after pretreatment with atropine; the inhibitory effect of L-NNA was more pronounced when distension of the rectal wall occurred close to the anal canal. L-arginine showed an opposite effect when compared with the effect of L-NNA, suppressing initial contraction of the descending response in the anal canal when rectal distension was applied next to the anal canal. In atropine-pretreated preparations, L-arginine extended distension-induced relaxation of the distal rectum by more than two-fold as compared with relaxation in the descending response when distension was applied to the proximal rectum (Figure 3).
Effects of TTX
The addition of TTX at a concentration of 0.1 μmol/L to the nutrient solution in the oral or anal compartments of the bath for 10 min prevented balloon distension-induced descending motor responses in the anal canal (n=4, data not shown).
ChAT– and NADPH-diaphorase-positive neuronal structures
ChAT-positive varicose nerve fibers were observed in the myenteric ganglia of the rectum (Figure 4A) and in the anal canal. Single immunoreactive nerve fibers running parallel to the muscle cells were present in both regions.
ChAT-immunoreactivity in myenteric ganglions (large arrow) of the anal canal. The arrows point to positive nerve fibers (A); NADPH-diaphorase-positive nerve cell bodies (arrowheads) in the myenteric ganglions (large arrow) and nerve fibers in the internodal strands (arrows) of the rectum (B); positive nerve cell bodies (arrowheads) showing high staining intensity for NADPH-diaphorase in the myenteric ganglions (large arrow) in the anal canal. Arrows point to nerve fibers running along internodal strands (C). Scale bar=50 μm.
Many NADPH-diaphorase-positive neurons, their processes and fibers were located in the myenteric ganglia of the rectum (Figure 4B) and the anal canal (Figure 4C). Single positive fibers in close proximity to the longitudinal muscles, large bundles between circular muscle cells and varicose nerve fibers were found between the muscle cells. The density and reaction intensity of NADPH-diaphorase-positive nerve structures were higher in the anal canal (+++) than in the rectum (+).
Discussion
The present study evaluated reflex evacuatory activity of the recto-anal region with respect to the anatomical and functional integrity of the anal sphincters. The balloon inflation-induced descending motor responses of the anal canal in the rat recto-anal model preparation were neurogenic in nature since they were prevented by TTX, an inhibitor of neuronal conductance. The partitioned organ bath method used in our experiments allowed for the evaluation of descending motor responses in the anal canal as induced by local rectal wall distension at different distances. Hence, we were able to characterize the physiological topography of locality-dependent activation of the descending reflex pathways involved in the integrative neuronal circuitry of the anal canal.
Rectal wall distension is a factor that influences the excitation of mechanoceptors that are involved in monitoring the filling state and the contraction level of the rectum in humans and cats24, 25. Detection of mechanical deformation by mechanoceptors containing specialized intraganglionic laminal endings, which are found in dense afferent innervations, has been described in the guinea pig rectum26, 27. Rectal content-induced stretching of the rectal wall is sufficient to initiate the defecation reflex in humans28. Our experiments show that, in the rat recto-anal region, the pattern of balloon-induced descending reflex motor responses in the anal canal depend on the position of distension-induced stretching of the rectal wall along the rectum. When rectal distension was applied at a distance away from the anal canal, the motor response that ensued was contraction. In contrast, when inflation of the rectal wall occurred at a distance close to the anal canal, the response was relaxation. These results suggest that the evacuatory mechanism reflex consists of a locality-dependent component. Changes in the pattern of motor responses in the anal canal most probably indicate that distension-activated mechanoceptors located along the rectum communicate with different neurotransmission(s) and act consecutively during the evacuation process.
The contractile responses or components of distension-induced descending responses in the anal canal decreased, whereas relaxation increased when the distance between the distension stimulus and the anal canal was shortened. This result demonstrates the prevalence of inhibitory neurotransmission(s) in the anal canal. Acetylcholine and substance P have been shown to be involved in excitatory enteric neurotransmission(s), whereas nitric oxide and VIP are proposed to act as inhibitory transmitters with significance in ascending or descending neural pathways, respectively5, 29, 30, 31, 32. We observed that distension-induced descending contractile responses or the contractile components of the responses in the anal canal were significantly decreased but not inhibited by atropine. This observation indicates that in the rat recto-anal region cholinergic and excitatory non-cholinergic neurotransmissions are involved in descending motor reflex pathways. Given that relaxation in the presence of atropine appeared or was increased in magnitude when previously present in distension-induced descending responses in the anal canal, we suggest that inactivation of excitatory cholinergic neurotransmission unmasked the action of inhibitory neurotransmission(s). Nitric oxide release from non-adrenergic, non-cholinergic nerves has been proposed to act as the main inhibitory mechanism in the recto-anal region14, 31, 33.
The present results demonstrate that nitric oxide-related drugs affect distension-induced descending motility of the anal canal. L-NNA, an inhibitor of nitric oxide synthase, increased contractions or contractile components and decreased relaxation before and during atropine treatment. In contrast, L-arginine, a substrate for nitric oxide synthesis, decreased contractile activity and increased relaxation. These observations are consistent with the view that nitric oxide can modulate neurotransmitter release from motor nerve endings. L-NNA-induced inhibition of nitric oxide synthesis prevents the direct inhibitory action of nitric oxide and leads to suppression of the negative modulation of acetylcholine release3. This effect results in a reduction in relaxation and in an extension of contractile activity in atropine-untreated anal canal preparations. In the guinea pig recto-anal region, L-NNA enhances reflex-mediated contraction of the rectum, abolishes reflex-mediated relaxation of the internal anal sphincter and converts the latter into a cholinergic contraction34. We observed that blocking cholinergic receptors allows for the manifestation of excitatory neurotransmitter(s) with the exception of cholinergic neurotransmitters. More recent data suggest that substance P is an excitatory mediator of the internal anal sphincter and that the increase in contractile components during anal canal responses to L-NNA treatment, in the presence of atropine, are attributed to the release of substance P during the recto-anal inhibitory reflex35. L-arginine decreased contraction and increased relaxation of the anal canal confirming the role of nitric oxide in the inhibitory neural pathways of the recto-anal region. The presence of NADPH-diaphorase-positive nerve structures in the myenteric ganglia and in the muscle coat of the rectum and anal canal indicates that there is a physiological significance for nitrergic neurotransmission during recto-anal motor activity. However, contractile and relaxation activity of distension-induced descending responses in the anal canal occurred in the presence of nitric-oxide-related drugs. This response indicates that other neuronal pathways, likely excitatory adrenergic and inhibitory peptidergic, may play a role in the modulation of anal canal motility4, 33, 32, 36.
We observed that the effects of nitrergic substances on reflex motor responses of the anal canal were dependent on the localization of rectal distension. The action of these substances was progressively increased when distension of the rectal wall moved in the aboral direction suggesting a higher density of distension-activated nitric oxide-dependent neural pathways in the anal part of the recto-anal region. The density of NADPH-diaphorase-positive neurons, processes and fibers was higher in the anal canal region. Nitric oxide-related drugs affected both contraction and relaxation of distension-induced descending reflex motor responses in the anal canal indicating that nitric oxide is an important contributor to the function of both excitatory and inhibitory descending reflex pathways in the neuronal network of the rat recto-anal region. These findings are not unexpected since recent studies have extended the role of the nitrergic system and have shown its involvement in neurally-regulated processes, such as in α-2 adrenoceptor-mediated effects37, uptake carrier systems38 and adenosine receptor activation39. The role of nitric oxide in interneuronal interactions has been previously investigated38 and, according to Vizi et al40, nitric oxide is an ideal mediator of nonsynaptic communications. This universal action of nitric oxide most probably influences the modulation of rat recto-anal evacuatory motor activity.
In summary, the partitioned organ bath method used in this study allowed us to record different patterns of distension-induced descending motor reflex responses in the anal canal. We observed that the motor responses of the anal canal were dependent on the localization of rectal wall distension which induced contraction or relaxation when away from or near to the anal canal, respectively. These findings demonstrate a locality-dependence of reflex motility in the anal sphincters. The different patterns of responses that we observed suggest that distension-activated mechanoceptors located along the rectal wall communicate with different neurotransmission(s) and act consecutively during the evacuatory process. It can be assumed that the physiological topography of the recto-anal descending motor reflex involves topical distribution of longer excitatory cholinergic and non-cholinergic pathways situated along the entire length of the rectum. Contraction and short inhibition of these responses are likely to be mediated mainly by nitric oxide-dependent pathways located predominantly in the anal area and underlie relaxation of the anal canal.
Author contribution
Radomir RADOMIROV and Christina IVANCHEVA designed the research; Radomir RADOMIROV and Christina IVANCHEVA performed the research; Christina IVANCHEVA and Polina PETKOVA-KIROVA analyzed the data; Dimitar ITZEV was responsible for performing morphological techniques; Radomir RADOMIROV wrote the manuscript.
References
Bharucha AE . Pelvic floor: anatomy and function. Neurogastroenterol Motil 2006; 18: 507–19.
Grider JR, Makhlouf GM . Colonic peristaltic reflex: identification of vasoactive intestinal peptide as mediator of descending relaxation. Am J Physiol Gastrointest Liver Physiol 1986; 251: G40–5.
Smith TK, McCarron SL . Nitric oxide modulates cholinergic reflex pathways to the longitudinal and circular muscle in the isolated guinea-pig distal colon. J Physiol 1998; 512: 893–906.
Matsufuji H, Yokoyama J . Neural control of the internal anal sphincter motility. J Smooth Muscle Res 2003; 39: 11–20.
Bian XC, Heffer LF, Gwynne RM, Bornstein JC, Bertrand PP . Synaptic transmission in simple motility reflex pathways excited by distension in guinea pig distal colon. Am Gastrointest Liver Physiol 2004; 287: 1017–27.
Van der Veek PP, Schots ED, Masclee AA . Effect of neurotensin on colorectal motor and sensory function in humans. Dis Colon Rectum 2004; 47: 210–8.
Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ . The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol 2002; 282: G443–9.
Law NM, Bharucha AE, Zinsmeister AR . Rectal and colonic distension elicit viscerovisceral reflexes in humans. Am J Physiol Gastrointest Liver Physiol 2002; 283: G384–9.
Malcolm A, Camilleri M . Coloanal motor coordination in association with high-amplitude colonic contractions after pharmacological stimulation. Am J Gastroenterol 2000; 95: 715–9.
Shafik A, Shafik AA, Ahmed I . Role of positive anorectal feedback in rectal evacuation: the concept of a second defecation reflex: the anorectal reflex. J Spinal Cord Med 2003; 26: 380–3.
Sangwan YP, Coller JA, Barrett RC, Murray JJ, Roberts PL, Schoetz DJ Jr . Distal rectoanal excitatory reflex: a reliable index of pudendal neuropathy? Dis Colon Rectum 1995; 38: 916–20.
Radomirov R, Ivancheva C, Brading AF, Itzev D, Rakovska A, Negrev N . Ascending and descending reflex mototr activity of recto-anal region-colinergic and nitrergic implications in a rat model. Brain Res Bull 2009; 79: 147–55.
Maggi CA, Patacchini R, Santicioli P, Theodorsson E, Meli A . Several neuropeptides determine the visceromotor response to capsaicin in the guinea-pig isolated ileal longitudinal muscle. Eur J Pharmacol 1988; 148: 43–9.
Stebing JF, Brading AF, Mortensen NJ . Nitric oxide and the rectoanal inhibitory reflex: retrograde neuronal tracing reveals a descending nitrergic rectoanal pathway in a guinea-pig model. Br J Surg 1996; 83: 493–8.
Zbar AP, Aslam M, Gold DM, Catzen C, Gosling A, Kmiot WA . Parameters of the rectoanal inhibitory reflex in patients with idiopathic fecal incontinence and chronic constipation. Dis Colon Rectum 1998; 41: 200–8.
De Lorijn F, de Jonge WJ, Wedel T, Vanderwinden JM, Benninga MA, Boeckxstaens GE . Interstitial cells of Cajal are involved in the afferent limb of the rectoanal inhibitory reflex. Gut 2005; 54: 1107–13.
Kojima Y, Nakagawa T, Katsui R, Fujii H, Nakajiama Y, Takaki M . A 5-HT4 agonist, mosapride, enhances intrinsic rectorectal and rectoanal reflexes after removal of extrinsic nerves in guinea pig. Am J Physiol Gastrointest Liver Physiol 2006; 289: 351–60.
Nagano M, Ishimizu Y, Saitoh S, Okada H, Fukuda H . The defecation reflex in rats: fundamental properties and the reflex center. Auton Neurosci 2004; 111: 48–56.
Brading AF, Ivancheva C, Radomirov R . Functional coordination of motor activity in colonic and recto-anal smooth muscles in rat experimental model. Methods Find Exp Clin Pharmacol 2008; 30: 201–7.
Ivancheva C, Radomirov R . Excitatory ascending and descending motor responses in the guinea pig small intestine: a comparative study of longitudinal and circular muscles by a triple bath method. Methods Find Exp Clin Pharmacol 2001; 23: 223–9.
Tallarida RJ, Murray RB . Manual of Pharmacological Calculation with Computer Programs. New York: Springer-Verlag; 1981. p1–150.
Hsu SM, Raine L, Fanger H . Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedure. J Histochem Cytochem 1981; 29: 577–80.
Sherer-Singler U, Vincent SR, Kimura H, McGeer EG . Demonstration of a unique population of neurons with NADPH-diaphorase histochemistry. J Neurosci Methods 1983; 9: 229–34.
Sun WM, Read NW, Prior A, Daly JA, Cheah SK, Grundy D . Sensory and motor responses to rectal distension vary according to rate and pattern of balloon inflation. Gastroenterology 1990; 99: 1008–15.
Ruhl A, Thewissen M, Ross HG, Cleveland S, Frieling T, Enck P . Discharge patterns of intramural mechanoreceptive afferents during selective distension of the cat's rectum. Neurogastroenterol Motil 1998; 10: 219–25.
Lynn PA, Zagorodnyuk V, Henning G, Costa M, Brookes S . Mechanical activation of rectal intraganglionic laminar endings in the guinea pig distal put. J Physiol 2005; 564: 589–601.
Zagorodnyuk VP, Lynn P, Costa M, Brookes SJ . Mechanisms of mechanotransduction by specialized low-threshold mechanorceptors in the guinea pig rectum. Am J Physiol Gastrointest Liver Physiol 289: 397–406..
Shafik A, Mostafa RM, Shafik I, Ei-Sibai O, Shafik AA . Functional activity of the rectum: A conduit organ or a storage organ or both? World J Gastroenterol 2006; 12: 4549–52.
Holzer P, Schluet W, Maggi CA . Ascending enteric reflex contraction: roles of acetylcholine and tachykinins in relation to distension and propagation of excitation. J Pharmacol Exp Ther 1993; 264: 391–6.
Johnson, PJ, Bornstein JC, Yuan SY, Furness JB . Analysis of contributions of acetylcholine and tachykinins to neuro-neuronal transmission in motility reflexes in the guinea-pig ileum. Br J Pharmacol 1996; 118: 973–83.
Rand MJ, Li CG . Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Annu Rev Physiol 1995; 57: 659–82.
Rattan S, Regan RF, Patel CA, De Godoy MA . Nitric oxide not carbon monoxide mediates nonadrenergic noncholinergic relaxation in the murine internal anal sphincter. Gastroenterology 2005; 129: 1954–66.
Cook TA, Brading AF, Mortensen NJ . The pharmacology of the internal anal sphincter and new treatments of ano-rectal disorders. Aliment Pharmacol Ther 2001; 15: 887–98.
Yamanouchi M, Shimatani H, Kadowaki M, Yoneda S, Nakagawa T, Fujii H, et al. Integrative control of rectoanal reflex in guinea pig through lumbar colonic nerves. Am J Physiol Gastrointest Liver Physiol 2002; 283: G148–56.
Yang G, Zhong T, Cheng WY, Ding GS . Change of substance P in portal vein during rectoanal inhibitory reflex. Zhonghua Wei Chang Wai Ke Za Zhi 2006; 9: 538–41.
Park JS, Kang SB, Kim DW, Namgung HW, Kim HL . The efficacy and adverse effects of topical phenylephrine for anal incontinence after low anterior resection in patients with rectal cancer. Int J Colorectal Dis 2007; 22: 1319–24.
Gyires K, Zadori ZS, Shujaa N, Minorics R, Falkay G, Matyus P . Analysis of the role of central and peripheral alpha2-adrenoceptor subtypes in gastric mucosal defense in the rat. Neurochem Int 2007; 51: 289–96.
Kiss JP, Vizi ES . Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci 2001; 24: 211–5.
Timoteo MA, Oliveira L, Campesato-Mella E, Barroso A, Silva C, Magalhaes-Cardoso MT, et al. Tuning adenosine A1 and A2A receptors mediates L-citrulline-induced inhibition of [3H]-acetylcholine release depending on nerve stimulation pattern. Neurochem Int 2008; 52: 834–45.
Vizi ES . Role of high-affinity receptors and membrane transporters in nonsynaptic communication and drug action in the central nervous system. Pharmacol Rev 2000; 52: 63–89.
Acknowledgements
This work was supported by a grant from the Bulgarian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radomirov, R., Ivancheva, C., Itzev, D. et al. Locality-dependent descending reflex motor activity in the anal canal–cholinergic and nitrergic contributions in the rat model. Acta Pharmacol Sin 30, 1276–1282 (2009). https://doi.org/10.1038/aps.2009.121
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2009.121