Abstract
Aim:
Stromelysin 1 (matrix metalloproteinase 3; MMP-3) is an enzyme known to be involved in tumor invasion and metastasis. In this study, flavonoids from vegetables and fruits, such as quercetin, kaempferol, genistein, genistin, and daidzein, were tested for their ability to modulate the secretion and activity of MMP-3 in the MDA-MB-231 breast cancer cell line. In addition, we investigated the in vitro effects of flavonoids on MDA-MB-231 cell invasion.
Methods:
The toxic concentration range of flavonoids was evaluated using the MTT assay. The ability of MDA-MB-231 cells to invade was evaluated using a modified Boyden chamber system. The activity of MMP-3 was determined by casein zymography. The secretion of MMP-3 was evaluated using Western blotting, casein zymography and confirmed by ELISA.
Results:
Some putative flavonoids, ie, quercetin and kaempferol (flavonols), significantly inhibited the in vitro invasion of MDA-MB-231 cells in a concentration-dependent manner, with IC50 values of 27 and 30 μmol/L, respectively. Quercetin and kaempferol also reduced MMP-3 activity in a dose-dependent manner, with IC50 values in the range of 30 μmol/L and 45 μmol/L, respectively. None of the flavonoids had a significant effect on the secretion of MMP-3.
Conclusion:
These data show that the flavonols quercetin and kaempferol have higher anti-invasion potency and higher MMP-3 inhibitory activity than isoflavones genistein, genistin and daidzein. In contrast, neither flavonols nor isoflavones have any effect on MMP-3 secretion.
Similar content being viewed by others
Introduction
Metastatic invasion is the primary cause of patient mortality during breast cancer progression. For a transformed cell to metastasize to a distant site in the body, it must first lose adhesion, penetrate and invade the surrounding extracellular matrix (ECM), enter the vascular system, and adhere to distant organs1. The inhibition of invasion of cancer cells has been an important strategy in cancer treatment2. A crucial step in the invasive processes is the proteolytic degradation of the ECM and basal membranes3. Several studies have shown that among the enzymes responsible for ECM degradation, the matrix metalloproteinases (MMPs) appear to play a critical role4, 5, 6. MMPs are zinc-dependent endopeptidases, which collectively can degrade all constituents of the ECM. Based on their structure and substrate specificity, they can be divided into subgroups of collagenases, stromelysins, gelatinases, membrane-type MMPs and other MMPs7.
Breast cancer is the major cause of malignancy-related deaths of women worldwide. In Thailand, breast cancer is the second most common cancer among women and its incidence is increasing8. Cancer invasion and metastasis are leading causes of morbidity and mortality in patients with breast cancer. Several studies have demonstrated that upregulation of MMP-1, -2, -3, -7, -9, -13, and -14 is associated with breast cancer cell invasion9, 10, 11.
MMP-3, also designated stromelysin 1, is a member of the matrixin family, which plays a pivotal role in the degradation and remodeling of the ECM. MMP-3 degrades several components of the ECM, such as fibronectin, laminin, collagen type IV12, 13, 14 and proteoglycans, and is thought to play an important role in rheumatoid arthritis, osteoarthritis15, 16, tumor cell invasion and metastasis17. Previous studies have demonstrated that the most invasive breast cancers exhibit the highest levels of MMP-3, while cells with an intermediate level of invasiveness have lower expression levels of MMP-3; noninvasive human breast cancer cells have undetectable levels of MMP-3. Recently, experimental evidence has shown that specific down-regulation of MMP-3 by anti-sense gene transfer leads to a loss of breast cancer invasion18. In addition, inhibition of MMP-3 activity with synthetic inhibitors reduces the invasion and migration of various human malignant cell lines19, 20. Therefore, every level of regulation of MMP-3 expression and activity has been considered as a possible target for therapeutic intervention21.
Flavonoids, 2-phenyl-benzo-α-pyrones, are polyphenolic compounds that occur ubiquitously in fruits, vegetables, and plant-derived beverages22. These compounds possess a common phenylbenzopyrone structure (C6-C3-C6), with one or more hydroxyl substituents (Figure 1). They are categorized (according to the saturation level and opening of the central pyran ring) primarily into flavonols, flavones, flavanols, isoflavones, flavonones, and flavanonols. These polyphenolic compounds have several biological activities, which include antimutagenic, antiproliferative and antioxidant effects, as well as involvement in cell signaling, cell cycle regulation and angiogenesis23. Furthermore, an increasing number of in vitro and in vivo studies have been conducted on the potential antimetastasis activity of flavonoids in various tumor cells including human breast cancer. For example, quercetin and genistein have been shown to reduce human breast cancer cell invasion via down-regulation of MMP-1, -2, and -9 expression24, 25, 26. In an in vivo study, intraperitoneal administration of quercetin into syngeneic mice resulted in significant inhibition of lung colonization in a dose-dependent manner27. Moreover, kaempferol, genistin and daidzein inhibited MDA-MB-231 cell invasion, with a concomitant reduction in the expression of ECM degradation enzymes28.
However, the correlation between MMP-3 and flavonoids in vitro remains unclear. In the present study we designed experiments to compare the effect of flavonoids in the form of flavonols (quercetin and kaempferol) and isoflavones (genistein, genistin and daidzein) on MMP-3 secretion, MMP-3 activity and invasive activity in MDA-MB-231 human invasive breast cancer cells. Our results demonstrate that the flavonols quercetin and kaempferol show higher anti-invasion potency and better MMP-3 inhibitory activity than isoflavones.
Materials and methods
Materials
Dulbecco's Modified Eagle's Medium (DMEM) with or without phenol red, penicillin-streptomycin, and trypsin-EDTA was purchased from GIBCO-BRL (Grand Island, NY, USA). Fetal bovine serum was purchased from Hyclone (Logan, Utah, USA). The antibody against MMP-3 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Matrigel was purchased from Becton Dickinson (Bedford, MA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), β-casein, quercetin, kaempferol, genistein, genistin and daidzein were purchased from Sigma-Aldrich (St Louis, MO, USA).
Cell lines and culture conditions
MDA-MB-231, a highly invasive breast carcinoma cell, was grown in Leibovitz L-15 medium with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated FBS. The cells were grown as a monolayer at 37 °C in a humidified atmosphere without CO2. MCF-7, a non-invasive breast carcinoma cell, and NIH3T3 fibroblasts cells were grown in DMEM supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated FBS. Cultures were grown as a monolayer at 37 °C in a 5% CO2/95% air atmosphere.
MTT assay for cell viability
Cell viability was measured by the conventional MTT reduction assay as previously described29. Briefly, MDA-MB-231 cells were inoculated at a density of 5×103 cells/well in 96-well plates for 24 h in 100 μL of DMEM with 10% FCS. Following seeding, the culture supernatant was removed and serum-free DMEM medium containing various concentrations of quercetin, kaempferol, genistein, genistin and daidzein was added and the cells were incubated for 24 and 48 h. MTT dye (10 μL, 5 mg/mL) was added to each well and the plate was incubated for an additional 4 h. The absorbance of MTT-formazan was measured using a microplate reader at 540 nm with a reference wavelength of 630 nm.
Cell invasion assay
The invasive behavior of MDA-MB-231 cells was tested using the modified Boyden chamber assay30. Polyvinylpyrrolidone-free polycarbonate filters (Millipore, Co Cork, Ireland) (8 μm pore sized) were coated with Matrigel (10 μg/filter). The lower chamber contained serum-free conditioned medium from NIH 3T3 fibroblast cells, which acts as a chemoattractant. MDA-MB-231 cells (1.25×105 cells/chamber) were plated onto the upper chamber with or without various concentrations of flavonoids and incubated for 6 h at 37 °C, 5% CO2. After incubation, the noninvading cells were removed from the upper surface of the membrane. The invading cells on the lower surface of the membrane were fixed with methanol for 1 min and stained with toluidine blue for 5 min. The cells that had actively migrated to the underside surface of the filter were dissolved with 20% acetic acid and indirectly quantified by measuring the absorbance at 570 nm. A control experiment was performed in the absence of chemoattractant. The results of duplicate independent experiments were averaged after background subtraction.
Preparation of conditioned media
MDA-MB-231 cells (1×106 cells) were seeded into a 75-mm3 T flask and maintained for 24 h in DMEM with 10% FBS. Subconfluent cell cultures were incubated for 48 h with various non-toxic concentrations of flavonoids in serum-free DMEM without phenol red. After treatment, the culture supernatant was collected and concentrated with Amicon-Ultra4 (Millipore, Co Cork, Ireland) for further studies.
Casein zymography
To determine MMP-3 activity in conditioned culture medium, MDA-MB-231 cells were incubated in serum-free DMEM without phenol red for 24 h at 37 °C in a 5% CO2 /95% air atmosphere. The culture supernatant was collected and concentrated with Amicon-Ultra4 (Millipore, Co Cork, Ireland). Ten micrograms of unheated protein from concentrated culture supernatants underwent electrophoresis under non-reducing conditions in 0.2% w/v casein-containing 10% polyacrylamide gels (PAGE) in the presence of SDS. Gels were washed twice for 60 min in 2.5% Triton X 100 to remove SDS and subsequently incubated in Tris buffer (50 mmol/L Tris-HCl, 200 mmol/L NaCl, 10 mmol/L CaCl2, pH 7.4) in the presence of various concentrations of flavonoids (0, 5, 25, and 50 μmol/L) and phenantroline 50 μmol/L (positive control) for 48 h at 37 °C. Gels were stained with Coomassie Brilliant Blue R (0.1% w/v) and destained in 30% methanol, 10% acetic acid. Caseinolytic activity appeared as a clear band on a blue background. Digestion bands were quantified by Bio 1 D software (Viber Lourmat, Marne-la-Vallée, France).
To evaluate MMP-3 secretion by casein zymography, MDA-MB-231 cells were incubated in serum-free DMEM without phenol red containing various concentrations of flavonoids (0, 10, 20, or 30 μmol/L). Ten micrograms of unheated protein from concentrated culture supernatants were subjected to electrophoresis under non-reducing conditions in 0.2% w/v casein-containing/10% polyacrylamide gels in the presence of SDS. Gels were washed, stained and destained as described above.
Western blot analysis of MMP-3
To investigate the effect of flavonoids on the secretion of MMP-3, Western blot analysis was performed. Equal amounts of concentrated conditioned media proteins from control and treated cells were resuspended in sample buffer and separated by SDS-PAGE, using 10% acrylamide gels. After electrophoresis, proteins were electroblotted to a Hybond–C Extra nitrocellulose membrane (Amersham). The membrane was blocked at room temperature (RT) with 4% BSA in TBST (20 mmol/L Tris pH 7.5, 150 mmol/L NaCl, 0.3% Tween 20) for 2 h. Membranes were further probed with mouse monoclonal antibody against MMP-3 in 4% BSA in TBST (1:200; Santa Cruz Biotechnology, USA) at 4 °C overnight. The blots were washed in TBST and probed with horseradish peroxidase conjugated anti-mouse IgG (1:5,000; Chemicon, France). After incubation, the immunoreactive material was visualized by enhanced chemiluminescence and exposed to X-ray film (Kodak, Windsor, Colorado, USA) for 30−60 min.
MMP-3 ELISA
To further confirm the effect of flavonoids on MMP-3 secretion, human MMP-3 double-sandwiched ELISA was performed using commercial ELISA kits according to the manufacturer's protocol (Calbiochem). Briefly, 50 μL standard dilutions of recombinant human MMP-3 or experimental conditioned media and biotinylated detector monoclonal antibody were aliquoted into a 96-well microtiter plate coated with mouse anti-MMP-3 monoclonal antibody. The plate was sealed, incubated at RT for 2 h, and washed three times with 1×washing buffer; 100 μL diluted horseradish peroxidase conjugated to streptavidin was added and incubated for 30 min at RT and subsequently washed three times. Aliquots of 100 μL of the color reagent 3,3′,5,5′-tetramethylbenzidine (TMB) were then applied for 30 min to develop a blue color, and the reaction was stopped by adding 100 μL of 2.5 mol/L sulfuric acid. Absorbance was read at 450 nm by a spectrophotometric plate reader with a reference wavelength of 595 nm.
Statistical analysis
Statistical analyses were performed using one-way ANOVA. P<0.05 or P<0.01 were considered statistically significant. All statistical analyses were performed using SPSS 13.0 software (SPSS, Chicago, IL, USA).
Results
In vitro cytotoxicity assay
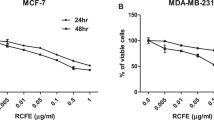
The effect of flavonoids on cell viability was measured by the MTT assay. MDA-MB-231 cells treated with the indicated amounts of quercetin, kaempferol and daidzein (0−100 μmol/L) for 48 h showed a concentration-dependent inhibition of cell proliferation. Genistein and genistin did not show pronounced effects on cell viability. After a 24 h incubation, MDA-MB-231 cells showed eighty percent viability when treated with bioflavonoids at concentrations below 60 μmol/L. The IC50 values at 48 h incubation calculated from the dose effect curve were 44.3±21.7, 60.0±16.3, >100, >100, and 65±15 μmol/L for quercetin, kaempferol, genistein, genistin and daidzein, respectively (Figure 2).
Effect of flavonoids on cell viability in MDA-MB-231 cells. Cells were exposed to the indicated amounts of each flavonoids (0, 6.25, 12.5, 25, 50, and 100 μmol/L) for 24 and 48 h. The number of viable cells was determined by MTT assay. Viable cells were expressed as a percentage of untreated control cultures for each line. Results represent the mean±SD of three different experiments performed in triplicates. Statistical analyses were performed using one-way ANOVA. bP<0.05, cP<0.01 vs 0 μmol/L.
Effect of flavonoids on invasion activity
Cell invasion assays were performed with non-cytotoxic concentrations (10, 20, and 30 μmol/L) of bioflavonoids. The invasive behavior of MDA-MB-231 was tested using the modified Boyden chamber assay. MDA-MB-231 cells have the ability to invade through Matrigel. MDA-MB-231 cells were treated with quercetin, kaempferol, genistein, genistin and daidzein (0, 10, 20, and 30 μmol/L) for 6 h. Quercetin and kaempferol significantly inhibited the ability of the MDA-MB-231 cells to penetrate the reconstituted basement membrane, with an inhibitory concentration at 50% of control (IC50) values of 27 and 30 μmol/L, respectively. In contrast, genistein, genistin and daidzein had minimal effects on cell invasion even at high concentrations (Figure 3).
Effect of flavonoids on MDA-MB-231 cells invasion. MDA-MB-231 cells were seeded onto a Matrigel-coated filter containing quercetin, kaempferol, genistein, genistin and daidzein as various indicated concentrations (0, 10, 20, and 30 μmol/L), incubated for 6 h in 37 °C. The cells that actively migrated to lower surface of filters were quantitated as described in material and methods. Invasion was expressed as a percentage of control. The data represent the mean±SD of three-independent experiments. Statistical analyses were performed using one-way ANOVA. bP<0.05, cP<0.01 vs 0 μmol/L.
Effect of flavonoids on activity of MMP-3 in MDA-MB-231 cell lines
Matrix metalloproteinases are key enzymes involved in the process of cancer cell invasion. Concentrated serum-free media revealed digested bands at 57 and 45 kDa, corresponding to proMMP-3 and active MMP-3, respectively (Figure 4). The activity of MMP-3 was reduced by quercetin and kaempferol with IC50 values of 30 and 45 μmol/L, respectively. In contrast, the activity of MMP-3 was unaffected by genistein, genistin and daidzein treatment.
Effect of flavonoids on the caseinolytic activity of MMP-3 as determined by the casein zymography assay. Condition media of MDA-MB-231 were collected and concentrated and subjected to electrophoresis. The gels were washed, incubated with various concentrations of flavonoids (0, 5, 25, 50 μmol/L) and phenantroline 50 μmol/L (positive control) for 48 h at 37 °C, stained and destained as described in Methods. Caseinolytic activity appeared as a clear band on a blue background. 1: Negative control; 2: 5 μmol/L; 3: 25 μmol/L; 4: 50 μmol/L; 5: phenantroline 50 μmol/L (positive control). The relative caseinolytic activity of MMP-3 were quantified by Bio 1D software (Viber Lourmat, Marne-la-Vallée, France), where the untreated group represented 100%. The data represent the mean±SD of three-independent experiments. Statistical analyses were performed using one-way ANOVA. bP<0.05, cP<0.01 vs 0 μmol/L.
Effect of flavonoids on secretion of MMP-3 in MDA-MB-231 cell lines
To elucidate the effect of flavonoids on MMP-3 secretion, Western blot analysis and casein zymography were carried out. MDA-MB-231 cells (1×106 cells) were incubated with quercetin, kaempferol, genistein, genistin and daidzein at 10, 20, 30 μmol/L for 48 h. The secretion of MMP-3 was unaffected by treatment with flavonoids (Figure 5). To confirm these results the experiment was repeated using ELISA (data not shown). For the three methods, all tested flavonoids had no effect on MMP-3 secretion.
Effect of flavonoids on MMP-3 secretion in MDA-MB-231 cell as determined by Western blot analysis. (A) MDA-MB-231 cells were treated with 0, 10, 20, and 30 μmol/L of quercetin, kaempferol, genistein, genistin and daidzein for 48 h, conditioned medium was collected and concentrated for MMP-3 determination. Equal amount of proteins was loaded (80 μg/lane). 1: Negative control; 2: 10 μmol/L; 3: 20 μmol/L; 4: 30 μmol/L and casein zymography. (B) Condition media of MDA-MB-231 were collected and concentrated and subjected to electrophoresis. The gels were washed, incubated with various concentrations of flavonoids (0, 10, 20, and 30 μmol/L) for 48 h at 37 °C, stained and destained as described in Methods. Caseinolytic activity appeared as a clear band on a blue background. 1: Negative control; 2: 10 μmol/L; 3: 20 μmol/L; 4: 30 μmol/L. Curcuminoids treatment at 0–15 μmol/L were used as a positive control. The band intensity of MMP-3 secretion by both assays was quantitated by Bio 1 D (Viber Lourmat, Marne-la-Vallée,France, where the untreated group represented 100%. The data represent the mean±SD of three-independent experiments. Statistical analyses were performed using one-way ANOVA.
Discussion
Invasiveness or migration through the extracellular matrix (ECM) is a fundamental property of malignant cancer cells. It has been shown that elevated expression of different MMPs is associated with different metastatic stages in the progression of various types of tumors31. We found that the level of MMP-3 in MDA-MB-231 (highly invasive breast cancer cells) was four-fold higher than that in MCF-7 (poorly invasive breast cancer cells), suggesting that the expression levels of MMP-3 correlate with the metastatic potential of cancer cells. Elevated levels of MMPs are generally thought to contribute to tumor progression. More recently it has become clear that a complex dual role in tumor progression exists for some MMPs, such as MMP-332. Conflicting results have been published regarding the role of MMP-3 in tumorigenesis and metastasis. A recent study showed an acceleration of the growth of lymphoma cells that constitutively express MMP-3 when the cells were injected intrathymically into mice33. In contrast, other studies have shown that genetic ablation of MMP-3 does not significantly affect tumor growth and metastasis in the MMTV-PyMT model34. The transcriptional changes of other MMPs and their specific inhibitors in the MMTV-PyMT model may complicate the metastatic outcome. Overall, these results suggest that the expression of a single metalloproteinase, stromelysin 1, is insufficient for the progression of mammary adenocarcinomas to an invasive and metastatic phenotype. However, matrix degradation by MMPs can alter the basic processes of cell proliferation and apoptosis.
The chemopreventive effect of the flavonoids, flavonols (quercetin and kaempferol) and isoflavones (genistein, genistin and daidzein) has been observed through the suppression of cell proliferation35, inhibition of angiogenesis36, inhibition of invasion37 and stimulation of apoptosis in breast carcinoma cells38. The inhibition of invasion of cancer cells is of great significance in cancer treatment. Little is known about the interaction of MMP-3 and flavonoids in human breast carcinoma cells. Therefore, we examined the effects of flavonoids on MMP-3 secretion, MMP-3 activity and invasive activity using MDA-MB-231 cells in our experiments.
This study examined the effect of flavonoids on the invasive behavior of MDA-MB-231 using the modified Boyden chamber assay. As shown in Figure 3, quercetin and kaempferol significantly inhibited the ability of the MDA-MB-231 cells to penetrate the reconstituted basement membrane. In contrast, genistein, genistin and daidzein did not significantly affect cell invasion at high concentrations, suggesting that the flavonols (quercetin and kaempferol) have higher anti-invasion potency than the isoflavones (genistein, genistin and daidzein). As MMP-3 has been reported to play a role during the migratory and invasive processes of cancer, it is necessary to determine whether flavonoids can inhibit the invasiveness of tumor cells. Our results help clarify whether flavonoids can inhibit MMP-3 enzyme activity in MDA-MB-231 cells. Flavonols (quercetin and kaempferol) inhibited the activity of MMP-3. In contrast, the activity of MMP-3 was unaffected by genistein, genistin and daidzein treatment (Figure 4). In addition, we found that genistein could inhibit MDA-MB-231 cell invasion, but to a lesser extent than quercetin or kaempferol (Figure 3). However, it seemed to have no effect on the activity of MMP-3 (Figure 4). Possible mechanisms of genistein-mediated inhibition of MDA-MB-231 invasion include down-regulation of the transcription of MMP-2, MMP-9, and MT1-MMP, and up-regulation of tissue inhibitor of metalloproteinase-1 (TIMP-1)39, 40. Furthermore, Magee and coworkers41 demonstrated that other phytoestrogens, including genistein, inhibited MDA-MB-231 cell invasion without affecting cell viability. Consistent with our findings, bioflavonoids used at the concentrations in the cell invasion assay (up to 30 μmol/L) had no effect on cell viability.
MMP proteolytic activity is regulated at three levels: transcription, proenzyme activation, and inhibition. We found that flavonoids (both flavonols and isoflavones) had no effect on MMP-3 secretion (Figure 5). In accordance with these results, quercetin and kaempferol markedly inhibited the caseinolytic activity of MMP-3 and inhibited breast cancer cell invasion, suggesting a relationship between the metastatic potential and MMP-3 activity. Indeed, batimastat (BB-94) and marimastat (BB-2516), two specific inhibitors of MMPs in clinical trials, have been found to reduce tumor metastasis42. Inostamycin, an inhibitor of cytidine 5′-diphosphate 1,2-diacyl-sn-glycerol (CDP-DG, inositol transferase), has been found to suppress the invasion of HSC-4 tongue carcinoma cells by reducing the gelatinolytic activities of pro-MMP-9 and pro-MMP-243. Considering that the dietary flavonols like quercetin and kaempferol are very safe and exhibit anticancer activities against a wide variety of tumors, we suggest that they can also be used to suppress the progression of the metastatic process in breast carcinoma cells by reducing MMP-3 activity.
Author contribution
Pornngarm LIMTRAKUL designed the research and provided financial support; Kanokkarn PHROMNOI and Supachai YODKEEREE performed experiments; Pornngarm LIMTRAKUL, Kanokkarn PHROMNOI, and Songyot ANUCHAPREEDA analyzed data; Pornngarm LIMTRAKUL and Kanokkarn PHROMNOI wrote the paper.
References
Hanahan D, Weinberg RA . The hallmarks of cancer. Cell 2000; 100: 57–70.
Liu J, Zhang X, Yang F, Li T, Wei D, Ren Y . Antimetastatic effect of a lipophilic ascorbic acid derivative with antioxidation through inhibition of tumor invasion. Cancer Chemother Pharmacol 2006; 57: 584–90.
Curran S, Murray GI . Matrix metalloproteinases in tumour invasion and metastasis. J Pathol 1999; 189: 300–8.
De CY, Szpirer C, Aly MS, Cassiman JJ, Eeckhout Y, Rousseau G . The gene for tissue inhibitor of metalloproteinases-2 is localized on human chromosome arm 17q25. Genomics 1992; 14: 782–4.
Coussens LM, Werb Z . Matrix metalloproteinases and the development of cancer. Chem Biol 1996; 3: 895–904.
Westermarck J, Kahari VM . Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J 1999; 13: 781–92.
Denys H, De WO, Nusgens B, Kong Y, Sciot R, Le AT, et al. Invasion and MMP expression profile in desmoid tumours. Br J Cancer 2004; 90: 1443–9.
Sriplung H, Wiangnon S, Sontipong S, Sumitsawan Y, Martin N . Cancer incidence trends in Thailand, 1989–2000. Asian Pac J Cancer Prev 2006; 7: 239–44.
Balduyck M, Zerimech F, Gouyer V, Lemaire R, Hemon B, Grard G, et al. Specific expression of matrix metalloproteinases 1, 3, 9 and 13 associated with invasiveness of breast cancer cells in vitro. Clin Exp Metastasis 2000; 18: 171–8.
Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP . Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem 1999; 274: 13066–76.
Hotary K, Li XY, Allen E, Stevens SL, Weiss SJ . A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev 2006; 20: 2673–86.
Murphy GJ, Murphy G, Reynolds JJ . The origin of matrix metalloproteinases and their familial relationships. FEBS Lett 1991; 289: 4–7.
Wu JJ, Lark MW, Chun LE, Eyre DR . Sites of stromelysin cleavage in collagen types II, IX, X, and XI of cartilage. J Biol Chem 1991; 266: 5625–8.
Siri A, Knauper V, Veirana N, Caocci F, Murphy G, Zardi L . Different susceptibility of small and large human tenascin-C isoforms to degradation by matrix metalloproteinases. J Biol Chem 1995; 270: 8650–4.
Okada Y, Takeuchi N, Tomita K, Nakanishi I, Nagase H . Immunolocalization of matrix metalloproteinase 3 (stromelysin) in rheumatoid synovioblasts (B cells): correlation with rheumatoid arthritis. Ann Rheum Dis 1989; 48: 645–53.
Firestein GS, Paine MM, Littman BH . Gene expression (collagenase, tissue inhibitor of metalloproteinases, complement, and HLA-DR) in rheumatoid arthritis and osteoarthritis synovium. Quantitative analysis and effect of intraarticular corticosteroids. Arthritis Rheum 1991; 34: 1094–105.
Kleiner DE Jr, Stetler-Stevenson WG . Structural biochemistry and activation of matrix metalloproteases. Curr Opin Cell Biol 1993; 5: 891–7.
Hegedus L, Cho H, Xie X, Eliceiri GL . Additional MDA-MB-231 breast cancer cell matrix metalloproteinases promote invasiveness. J Cell Physiol 2008; 216: 480–5.
Heikkil P, Teronen O, Moilanen M, Konttinen YT, Hanemaaijer R, Laitinen M, et al. Bisphosphonates inhibit stromelysin-1 (MMP-3), matrix metalloelastase (MMP-12), collagenase-3 (MMP-13) and enamelysin (MMP-20), but not urokinase-type plasminogen activator, and diminish invasion and migration of human malignant and endothelial cell lines. Anti-Cancer Drugs 2002; 13: 245–54.
Mercapide J, Lopez De Cicco R, Castresana JS, Klein-Szanto AJ . Stromelysin-1/matrix metalloproteinase-3 (MMP-3) expression accounts for invasive properties of human astrocytoma cell lines. Int J Cancer 2003; 106: 676–82.
Yodkeeree S, Chaiwangyen W, Garbisa S, Limtrakul P . Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit cancer cell invasion through the down-regulation of MMPs and uPA. J Nutr Biochem 2009; 20: 87–95.
Heim KE, Tagliaferro AR, Bobilya DJ . Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 2002; 13: 572–84.
Shun FY, Wen EY, Wu HK, Horng RC, Shu CC, Yih SH . Antimetastatic potentials of flavones on oral cancer cell via an inhibition of matrix-degrading proteases. Arch Oral Biol 2008; 53: 287–94.
Lim H, Kim HP . Inhibition of mammalian collagenase, matrix metalloproteinase-1, by naturally-occurring flavonoids. Planta Med 2007; 73: 1267–74.
Lin CW, Hou WC, Shen SC, Juan SH, Ko CH, Wang LM, et al. Quercetin inhibition of tumor invasion via suppressing PKC delta/ERK/AP-1-dependent matrix metalloproteinase-9 activation in breast carcinoma cells. Carcinogenesis 2008; 29: 1807–15.
Vijayababu MR, Arunkumar A, Kanagaraj P, Venkataraman P, Krishnamoorthy G, Arunakaran J . Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3). Mol Cell Biochem 2006; 287: 109–16.
Caltagirone S, Rossi C, Poggi A, Ranelletti FO, Natali PG, Brunetti M, et al. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer 2000; 87: 595–600.
Lee WJ, Chen WK, Wang CJ, Lin WL, Tseng TH . Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol 2008; 226: 178–91.
Mosmann T . Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65: 55–63.
Dona M, Dell'Aica I, Pezzato E, Sartor L, Calabrese F, Della BM, et al. Hyperforin inhibits cancer invasion and metastasis. Cancer Res 2004; 64: 6225–32.
Airola K, Reunala T, Salo S, Saarialho-Kere UK . Urokinase plasminogen activator is expressed by basal keratinocytes before interstitial collagenase, stromelysin-1, and laminin-5 in experimentally induced dermatitis herpetiformis lesions. J Invest Dermatol 1997; 108: 7–11.
Martin MD, Matrisian LM . The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev 2007; 26: 717–24.
Van Themsche C, Potworowski EF, St-Pierre Y . Stromelysin-1 (MMP-3) is inducible in T lymphoma cells and accelerates the growth of lymphoid tumors in vivo. Biochem Biophys Res Commun 2004; 315: 884–91.
Juncker-Jensen A, Romer J, Pennington CJ, Lund LR, Almholt K . Spontaneous metastasis in matrix metalloproteinase 3-deficient mice. Mol Carcinog 2009; 48: 618–25.
Haddad AQ, Venkateswaran V, Viswanathan L, Teahan SJ, Fleshner NE, Klotz LH . Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis 2006; 9: 68–76.
Farina HG, Pomies M, Alonso DF, Gomez DE . Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol Rep 2006; 16: 885–91.
Zhang XM, Huang SP, Xu Q . Quercetin inhibits the invasion of murine melanoma B16-BL6 cells by decreasing pro-MMP-9 via the PKC pathway. Cancer Chemother Pharmacol 2004; 53: 82–8.
Rowell C, Carpenter DM, Lamartiniere CA . Chemoprevention of breast cancer, proteomic discovery of genistein action in the rat mammary gland. J Nutr 2005; 135: 2953S–2959S.
Magee PJ, McGlynn H, Rowland IR . Differential effects of isoflavones and lignans on invasiveness of MDA-MB-231 breast cancer cells in vitro. Cancer Lett 2004; 208: 35–41.
Kousidou OC, Mitropoulou TN, Roussidis AE, Kletsas D, Theocharis AD, Karamanos NK . Genistein suppresses the invasive potential of human breast cancer cells through transcriptional regulation of metalloproteinases and their tissue inhibitors. Int J Oncol 2005; 26: 1101–9.
Magee PJ, McGlynn H, Rowland IR . Differential effects of isoflavones and lignans on invasiveness of MDA-MB-231 breast cancer cells in vitro. Cancer Lett 2004; 208: 35–41.
Rasmussen HS, McCann PP . Matrix metalloproteinase inhibition as a novel anticancer strategy: a review with special focus on batimastat and marimastat. Pharmacol Ther 1997; 75: 69–75.
Baba Y, Tsukuda M, Mochimatsu I, Furukawa S, Kagata H, Nagashima Y, et al. Inostamycin, an inhibitor of cytidine 5′-diphosphate 1,2-diacyl-sn-glycerol (CDP-DG): inositol transferase, suppresses invasion ability by reducing productions of matrix metalloproteinase-2 and -9 and cell motility in HSC-4 tongue carcinoma cell line. Clin Exp Metastasis 2000; 18: 273–9.
Acknowledgements
This research was supported by grants from the National Research Council of Thailand (NRCT). Kanokkarn Promnoi and Supachai Yodkeeree are PhD students under the Royal Golden Jubilee PhD Program of Thailand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phromnoi, K., Yodkeeree, S., Anuchapreeda, S. et al. Inhibition of MMP-3 activity and invasion of the MDA-MB-231 human invasive breast carcinoma cell line by bioflavonoids. Acta Pharmacol Sin 30, 1169–1176 (2009). https://doi.org/10.1038/aps.2009.107
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2009.107
Keywords
This article is cited by
-
The Potential Clinical Uses and Nanoformulation Strategies of Kaempferol, a Dietary Flavonoid
Revista Brasileira de Farmacognosia (2022)
-
The potential health benefits of the isoflavone glycoside genistin
Archives of Pharmacal Research (2020)
-
Flavonoid calycopterin triggers apoptosis in triple-negative and ER-positive human breast cancer cells through activating different patterns of gene expression
Naunyn-Schmiedeberg's Archives of Pharmacology (2020)
-
Mannich aminomethylation of flavonoids and anti-proliferative activity against breast cancer cell
Chemical Papers (2018)
-
Arsenic-Induced Hepatic Toxicity and Its Attenuation by Fruit Extract of Emblica officinalis (Amla) in Mice
Indian Journal of Clinical Biochemistry (2014)