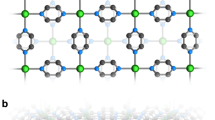
Abstract
Metallic behaviour is well known in charge-transfer complexes that contain stacks of planar, partially oxidized (or reduced) π-conjugated molecules. Electronic conduction occurs in the partially occupied, delocalized π bands formed by intermolecular orbital overlap, and some of these materials exhibit superconductivity1,2. Counter-ions, present to achieve charge neutrality, usually play a passive role, although in some cases they couple to the electronic structure, for example by imposing a new structural periodicity (a superlattice) by orientational ordering1. The development of molecular solids that can simultaneously support the transport of both electrons and ions is important for several fields, including the development of solid-state batteries3,4, electroluminescent devices5 and biomimetic systems6,7. Crown ethers are promising components for such systems, as they provide cavities through which ion motion might occur. Here we report that the charge-transfer salt Li0.6(15-crown-5-ether)[Ni(dmit)2]2·H2O exhibits both electron and ion conductivity: the stacks of the nickel complex (dmit is an organic molecule) provide a pathway for electron conduction, and stacks of the crown ethers provide channels for lithium-ion motion. Evidence for the latter above 250 K is provided by NMR and conductivity studies. We also see evidence for coupling of the electron and ion motions. This compound might serve as a model for the development of other hybrid electronic/ionic conducting materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jérome, D. The physics of organic superconductors. Science 252, 1509–1515 (1991).
Williams, J. M. et al. Organic superconductors—new benchmarks. Science 252, 1501–1508 (1991).
Takahashi, T. et al. Solid-state ionics—recent trends and future expectations. Bull. Electrochem. 11, 1–33 (1995).
Fauteux, D., Massucco, A., Mclin, M., Vanburen, M. & Shi, J. Lithium polymer electrolyte rechargeable battery. Electrochim. Acta 40, 2185–2190 (1995).
Pei, Q., Yu, G., Zhang, C., Yang, Y. & Heeger, A. J. Polymer light-emitting electrochemical cells. Science 269, 1086–1089 (1995).
Pregel, M. J., Jullien, L. & Lehn, J. M. Towards artificial ion channels—transport of alkali-metal ions across liposomal membranes by bouquet molecules. Angew. Chem. Int. Edn Engl. 31, 1637–1640 (1992).
Ghadiri, M. R., Granja, J. R. & Buehler, L. K. Artificial transmembrane ion channels from self-assembling peptide nanotubes. Nature 369, 301–304 (1994).
Cassoux, P. et al. Molecular-metals and superconductors derived from metal-complexes of 1,3-dithiol-2-thione-4,5-dithiolate (dmit). Coord. Chem. Rev. 110, 115–160 (1991).
Kim, H., Kobayashi, A., Sasaki, Y., Kato, R. & Kobayashi, H. New radical-anion complex, [(CH3)4N][Ni(dmit)2]2] with metal-semimetal phase-transition. Chem. Lett. 1799–1802 (1987).
Guy, D. R. P. & Friend, R. H. Temperature measurement in high pressure cells using a rhodium + 0.5% iron versus chromel thermocouple pair. J. Phys. E 19, 430–433 (1986).
Murata, K. et al. Superconductivity with the onset at 8 K in the organic conductor beta-(BEDT-TTF)2I3under pressure. J. Phys. Soc. Jpn 54, 1236–1239 (1985).
Estes, W. E., Gavel, D. P., Hatfield, W. E. & Hodgson, D. J. Magnetic and structural characterisation of dibromo- and dichlorobis(thiazole) copper(II). Inorg. Chem. 17, 1415–1421 (1978).
Obertelli, S. D., Friend, R. H., Talham, D. R., Kurmoo, M. & Day, P. Magnetic susceptibility and EPR of the Organic conductors α′-(BEDT-TTF)2X, X=AuBr2, CuCl2and Ag (CN)2. J. Phys. Cond. Matter 1, 5671–5680 (1989).
Cohen, M. H. & Reif, F. in Solid State Physics (eds Seitz, F. & Turnbull, D.) 321–438 (Academic, New York, (1957)).
Robitaille, C. D. & Fauteux, D. Phase-diagrams and conductivity characterization of some PEO-Lixelectrolytes. J. Electrochem. Soc. 133, 315–325 (1986).
Grimaldi, J. J. & Lehn, J. M. Multicarrier transport: coupled transport of electrons and metal cations mediated by an electron carrier and a selective cation carrier. J. Am. Chem. Soc. 101, 1333–1334 (1979).
Burla, M. C. et al. SIR88—a direct-methods program for the automatic solution of crystal-structures. J. Appl. Crystallogr. 22, 389–393 (1989).
Acknowledgements
We thank N. Robertson for synthesis of some of the crystals used in this work, N.Nonose for measurements of the ICP mass spectra, and A. Yap and S. R. Elliott for assistance with the NMR measurements. This work was supported by Grant-in-aid for Science Research from the Ministry of Education, Science and Culture, Japan, and the UK Engineering and Physical Sciences Research Council. One of the authors (A.E.U.) acknowledges support from the British Council.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Rights and permissions
About this article
Cite this article
Nakamura, T., Akutagawa, T., Honda, K. et al. A molecular metal with ion-conducting channels. Nature 394, 159–162 (1998). https://doi.org/10.1038/28128
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/28128
This article is cited by
-
Tuning the charge flow between Marcus regimes in an organic thin-film device
Nature Communications (2019)
-
Syntheses, electrochemical behaviors, spectral properties and DFT calculations of two 1,3-dithiole derivatives
Chemical Research in Chinese Universities (2014)
-
Ferroelectricity and polarity control in solid-state flip-flop supramolecular rotators
Nature Materials (2009)
-
Synthesis and crystal structure of catena-poly[aqua(cis-syn-cis-dicyclohexyl-18-crown-6) tetra(thiocyanato)barium(II) platinum(II)]: [BaPt(NCS)4(C20H36O6)(H2O)] n
Journal of Chemical Crystallography (2006)
-
Structure of Dibenzocrown Ethers and their H-Bonded Adducts.1. [1.5]Dibenzo-18-Crown-6 and Biphenyl-20-Crown-6: Complexes with Amidosulfuric Acid
Journal of Inclusion Phenomena and Macrocyclic Chemistry (2005)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.