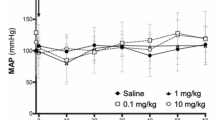
Abstract
Antiangiogenic gene therapy is a promising strategy for cancer treatment, which generally requires highly efficient delivery systems. To date, success of this strategy has depended almost exclusively on the delivery of high titers of viral vectors, which can result in effective transgene expression. However, their cytotoxicity and immunogenicity are a major concern for clinical applications. Recent advances in delivery efficiency of naked DNA could potentially meet the requirement for both high transgene expression and minimal side effects. To investigate whether naked DNA can be used for antiangiogenic cancer therapy, an expression plasmid was generated that encodes a soluble form of fetal liver kinase-1 (Flk-1) gene, a receptor for vascular endothelial growth factor (VEGF). Hydrodynamic injection of this plasmid resulted in close to 0.1 mg/ml of soluble Flk-1 protein in mouse serum and blocked VEGF-driven angiogenesis in matrigel in vivo. The same delivery significantly suppressed the growth of two different pre-existing subcutaneous tumors, Renca renal cell carcinoma and 3LL lung carcinoma. CD31 immunohistochemistry revealed that the tumor-associated angiogenesis was also highly attenuated in soluble Flk-1-treated mice. Thus, expression of genes by hydrodynamics-based gene delivery of naked DNA appears to be a promising approach for antiangiogenic cancer gene therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Folkman J . Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1: 27–31.
Boehm T, Folkman J, Browder T, O'Reilly MS . Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 1997; 390: 404–407.
Tang DG, Conti CJ . Endothelial cell development, vasculogenesis, angiogenesis, and tumor neovascularization: an update. Semin Thromb Hemost 2004; 30: 109–117.
Mitra A, Mulholland J, Nan A, McNeill E, Ghandehari H, Line BR . Targeting tumor angiogenic vasculature using polymer-RGD conjugates. J Control Release 2005; 20: 191–201.
Dickerson EB, Akhtar N, Steinberg H, Wang ZY, Lindstrom MJ, Padilla ML et al. Enhancement of the antiangiogenic activity of interleukin-12 by peptide targeted delivery of the cytokine to alphavbeta3 integrin. Mol Cancer Res 2004; 2: 663–673.
Yoshimura I, Mizuguchi Y, Miyajima A, Asano T, Tadakuma T, Hayakawa M . Suppression of lung metastasis of renal cell carcinoma by the intramuscular gene transfer of a soluble form of vascular endothelial growth factor receptor I. J Urol 2004; 171: 2467–2470.
Huang J, Frischer JS, Serur A, Kadenhe A, Yokoi A, McCrudden KW et al. Regression of established tumors and metastases by potent vascular endothelial growth factor blockade. Proc Natl Acad Sci USA 2003; 100: 7785–7790.
Bocci G, Man S, Green SK, Francia G, Ebos JM, du Manoir JM et al. Increased plasma vascular endothelial growth factor (VEGF) as a surrogate marker for optimal therapeutic dosing of VEGF receptor-2 monoclonal antibodies. Cancer Res 2004; 64: 6616–6625.
Huh JI, Calvo A, Stafford J, Cheung M, Kumar R, Philp D et al. Inhibition of VEGF receptors significantly impairs mammary cancer growth in C3(1)/Tag transgenic mice through antiangiogenic and non-antiangiogenic mechanisms. Oncogene 2005; 24: 790–800.
Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C et al. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell 2003; 3: 589–601.
Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R et al. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci USA 2001; 98: 4605–4610.
Takayama K, Ueno H, Nakanishi Y, Sakamoto T, Inoue K, Shimizu K et al. Suppression of tumor angiogenesis and growth by gene transfer of a soluble form of vascular endothelial growth factor receptor into a remote organ. Cancer Res 2000; 60: 2169–2177.
Davidoff AM, Nathwani AC, Spurbeck WW, Ng CYC, Zhou J, Vanin EF . rAAV-mediated long-term liver-generated expression of an angiogenesis inhibitor can restrict renal tumor Growth in Mice. Cancer Res 2002; 62: 3077–3083.
Mahendra G, Kumar S, Isayeva T, Mahasreshti PJ, Curiel DT, Stockardt CR et al. Antiangiogenic cancer gene therapy by adeno-associated virus 2-mediated stable expression of the soluble FMS-like tyrosine kinase-1 receptor. Cancer Gene Ther 2005; 12: 26–34.
Drittanti L, Jenny C, Poulard K, Samba A, Manceau P, Soria N et al. Optimised helper virus-free production of high-quality adeno-associated virus vectors. J Gene Med 2001; 3: 59–71.
Wells DJ . Gene therapy progress and prospects: electroporation and other physical methods. Gene Therapy 2004; 11: 1363–1369.
Acsadi G, Dickson G, Love DR, Jani A, Walsh FS, Gurusinghe A et al. Human dystrophin expression in mdx mice after intramuscular injection of DNA constructs. Nature 1991; 352: 815–818.
Zhang G, Budker V, Wolff JA . High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther 1999; 10: 1735–1737.
Zhang G, Song YK, Liu D . Long-term expression of human alpha1-antitrypsin gene in mouse liver achieved by intravenous administration of plasmid DNA using a hydrodynamics-based procedure. Gene Therapy 2000; 7: 1344–1349.
Fong CL, Hui KM . Generation of potent and specific cellular immune responses via in vivo stimulation of dendritic cells by pNGVL3-hFLex plasmid DNA and immunogenic peptides. Gene Therapy 2002; 9: 1127–1138.
Higuchi N, Maruyama H, Kuroda T, Kameda S, Iino N, Kawachi H et al. Hydrodynamics-based delivery of the viral interleukin-10 gene suppresses experimental crescentic glomerulonephritis in Wistar–Kyoto rats. Gene Therapy 2003; 10: 1297–1310.
Itokawa Y, Mazda O, Ueda Y, Kishida T, Asada H, Cui FD et al. Interleukin-12 genetic administration suppressed metastatic liver tumor unsusceptible to CTL. Biochem Biophys Res Commun 2004; 20: 1072–1079.
Chang J, Sigal LJ, Lerro A, Taylor J . Replication of the human hepatitis delta virus genome is initiated in mouse hepatocytes following intravenous injection of naked DNA or RNA sequences. J Virol 2001; 75: 3469–3473.
Yang PL, Althage A, Chung J, Chisari FV . Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc Natl Acad Sci USA 2002; 99: 13825–13830.
Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med 2003; 9: 347–351.
Zender L, Hutker S, Liedtke C, Tillmann HL, Zender S, Mundt B et al. Caspase 8 small interfering RNA prevents acute liver failure in mice. Proc Natl Acad Sci USA 2003; 100: 7797–7802.
Hagstrom JE, Hegge J, Zhang G, Noble M, Budker V, Lewis DL et al. A facile nonviral method for delivering genes and siRNAs to skeletal muscle of mammalian limbs. Mol Ther 2004; 10: 386–398.
Zhang G, Ludtke JJ, Thioudellet C, Kleinpeter P, Antoniou M, Herweijer H et al. Intraarterial delivery of naked plasmid DNA expressing full-length mouse dystrophin in the mdx mouse model of duchenne muscular dystrophy. Hum Gene Ther 2004; 15: 770–782.
Ferrara N . Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol 1999; 237: 1–30.
Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z . Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999; 13: 9–22.
Warren RS, Yuan H, Matli MR, Gillett NA, Ferrara N . Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest 1995; 95: 1789–1797.
Borgstrom P, Hillan KJ, Sriramarao P, Ferrara N . Complete inhibition of angiogenesis and growth of microtumors by anti-vascular endothelial growth factor neutralizing antibody: novel concepts of angiostatic therapy from intravital videomicroscopy. Cancer Res 1996; 56: 4032–4039.
Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE . Halting angiogenesis suppresses carcinoma cell invasion. Nat Med 1997; 3: 1222–1227.
Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993; 362: 841–844.
Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res 1999; 59: 99–106.
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003; 349: 427–434.
Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004; 10: 145–147.
Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003; 21: 60–65.
Liu F, Song Y, Liu D . Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Therapy 1999; 6: 1258–1266.
Yew NS, Wang KX, Przybylska M, Bagley RG, Stedman M, Marshall J et al. Contribution of plasmid DNA to inflammation in the lung after administration of cationic lipid:pDNA complexes. Hum Gene Ther 1999; 10: 223–234.
He Y, Pimenov AA, Nayak JV, Plowey J, Falo Jr LD, Huang L . Intravenous injection of naked DNA encoding secreted flt3 ligand dramatically increases the number of dendritic cells and natural killer cells in vivo. Hum Gene Ther 2000; 11: 547–554.
Hoffmann J, Schirner M, Menrad A, Schneider MR . A highly sensitive model for quantification of in vivo tumor angiogenesis induced by alginate-encapsulated tumor cells. Cancer Res 1997; 57: 3847–3851.
Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res 1997; 57: 963–969.
Bockhorn M, Tsuzuki Y, Xu L, Frilling A, Broelsch CE, Fukumura D . Differential vascular and transcriptional responses to anti-vascular endothelial growth factor antibody in orthotopic human pancreatic cancer xenografts. Clin Cancer Res 2003; 9: 4221–4226.
Fortuin FD, Vale P, Losordo DW, Symes J, DeLaria GA, Tyner JJ et al. One-year follow-up of direct myocardial gene transfer of vascular endothelial growth factor-2 using naked plasmid deoxyribonucleic acid by way of thoracotomy in no-option patients. Am J Cardiol 2003; 92: 436–439.
Kim HJ, Jang SY, Park JI, Byun J, Kim DI, Do YS et al. Vascular endothelial growth factor-induced angiogenic gene therapy in patients with peripheral artery disease. Exp Mol Med 2004; 36: 336–344.
Kong HL, Hecht D, Song W, Kovesdi I, Hackett NR, Yayon A et al. Regional suppression of tumor growth by in vivo transfer of a cDNA encoding a secreted form of the extracellular domain of the flt-1 vascular endothelial growth factor receptor. Hum Gene Ther 1998; 9: 823–833.
Prewett M, Huber J, Li Y, Santiago A, O'Connor W, King K et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res 1999; 59: 5209–5218.
Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S . Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol 2005; 23: 3706–3712.
Wolff JA, Herweijer H . Nonviral vectors for cardiovascular gene delivery. Ernst Schering Res Found Workshop 2003; 10: 41–59.
Zhang G, Gao X, Song YK, Vollmer R, Stolz DB, Gasiorowski JZ et al. Hydroporation as the mechanism of hydrodynamic delivery. Gene Therapy 2004; 11: 675–682.
Kobayashi N, Nishikawa M, Takakura Y . The hydrodynamics-based procedure for controlling the pharmacokinetics of gene medicines at whole body, organ and cellular levels. Adv Drug Deliv Rev 2005; 57: 713–731.
Herweijer H, Zhang G, Subbotin VM, Budker V, Williams P, Wolff JA . Time course of gene expression after plasmid DNA gene transfer to the liver. J Gene Med 2001; 3: 280–291.
Zhang G, Budker V, Williams P, Subbotin V, Wolff JA . Efficient expression of naked DNA delivered intraarterially to limb muscles of nonhuman primates. Hum Gene Ther 2001; 12: 427–438.
Eastman SJ, Baskin KM, Hodges BL, Chu Q, Gates A, Dreusicke R et al. Development of catheter-based procedures for transducing the isolated rabbit liver with plasmid DNA. Hum Gene Ther 2002; 20: 2065–2077.
Streit M, Stephen AE, Hawighorst T, Matsuda K, Lange-Asschenfeldt B, Brown LF et al. Systemic inhibition of tumor growth and angiogenesis by thrombospondin-2 using cell-based antiangiogenic gene therapy. Cancer Res 2002; 62: 2004–2012.
Yao L, Pike SE, Setsuda J, Parekh J, Gupta G, Raffeld M et al. Effective targeting of tumor vasculature by the angiogenesis inhibitors vasostatin and interleukin-12. Blood 2000; 96: 1900–1905.
Vajkoczy P, Thurnher A, Hirth KP, Schilling L, Schmiedek P, Ullrich A et al. Measuring VEGF-Flk-1 activity and consequences of VEGF-Flk-1 targeting in vivo using intravital microscopy: clinical applications. Oncologist 2000; 5 (Suppl 1): 16–19.
Kusters B, de Waal RMW, Wesseling P, Verrijp K, Maass C, Heerschap A et al. Differential effects of vascular endothelial growth factor a isoforms in a mouse brain metastasis model of human melanoma. Cancer Res 2003; 63: 5408–5413.
Acknowledgements
We thank Dr John Ortaldo and Dr Jonathan Weiss for their critical review of this manuscript, the NCI, CCR Fellows Editorial Board for their editing, and Ms Susan Charbonneau and Ms Linda Grove for her excellent secretarial work. This work was partly supported by the Foreign Research Fellow Program funded by the Japanese Ministry of Education, Culture, Sports, Science and Technology. This project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. N01-CO-12400.
The publisher or recipient acknowledges the right of the US Government to retain a non-exclusive, royalty-free license in and to any copyright covering the article.
Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication no. 86–23, 1985).
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yazawa, H., Murakami, T., Li, HM. et al. Hydrodynamics-based gene delivery of naked DNA encoding fetal liver kinase-1 gene effectively suppresses the growth of pre-existing tumors. Cancer Gene Ther 13, 993–1001 (2006). https://doi.org/10.1038/sj.cgt.7700970
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700970
Keywords
This article is cited by
-
Hydrodynamic delivery of plasmid DNA encoding human FcγR-Ig dimers blocks immune-complex mediated inflammation in mice
Gene Therapy (2012)
-
Hydrodynamic Gene Delivery and Its Applications in Pharmaceutical Research
Pharmaceutical Research (2011)
-
Gene Transfer: How Can the Biological Barriers Be Overcome?
The Journal of Membrane Biology (2010)
-
Hydrodynamic Gene Delivery: Its Principles and Applications
Molecular Therapy (2007)