Abstract
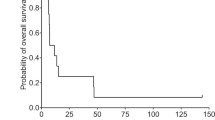
ONYX-015 is an adenovirus that selectively replicates in p53 dysfunctional or mutated malignant cells. We performed a pilot trial to determine the safety and feasibility of treatment with ONYX-015 delivered intravenously in patients with advanced malignancy. One cohort of five patients received ONYX-015 once a week for 6 weeks at a dose of 2 × 1012 particles per infusion in combination with weekly infusions of irinotecan (CPT11, 125 mg per week) and 5-fluorouracil (5FU, 500 mg per week). A second cohort of five patients received the combination of ONYX-015 at a dose of 2 × 1011 particles per week for 6 weeks in combination with interleukin 2 (IL 2, 1.1 × 106 units daily via subcutaneous injection for 5 days each week for 4 weeks). Toxicity attributable to ONYX-015 was limited to transient fever. All patients demonstrated elevations in neutralizing antibody titers within 4 weeks of the infusion of ONYX-015. Serum levels of IL-6, IL-10, tumor necrosis factor-α, and interferon-γ increased within 6 hours of viral infusion, suggesting immune activation. This response was more pronounced in the cohort of patients who received 2 × 1012 particles per infusion. Two patients demonstrated uptake of viral particles in malignant tissue by quantitative PCR. Electron microscopy confirmed selective cytoplasmic viral particles within malignant cells but not within adjacent normal tissue in a third patient. In conclusion ONYX-015 can be administered safely in combination with CPT11, 5FU or low-dose IL 2 and is able to access malignant tissue following intravenous infusion. Further investigation of ONYX-015, possibly with agents that may modulate replication activity, or duration of virus survival, is indicated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Heise C, Kirn D . Replication-sensitive adenoviruses for cancer. J Clin Invest. 2000;105:847–851.
Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376.
Heise C, et al. Intravenous administration of ONYX-015, a replication-selective adenovirus, induces anti-tumoral efficacy. Cancer Res. 1999;59:2623–2628.
Heise C, et al. ONYX-015, and E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and anti-tumoral efficacy that can be augmented by standard chemotherapeutic agents. Nature Med. 1997;3:639–645.
Fujiwara T, et al. Induction of chemosensitivity in human lung cancer cells in vivo by adenovirus-mediated transfer of the wild-type p53 gene. Cancer Res. 1994;54:2287–2291.
You L, Yang C-T, Jablons DM . ONYX-015 works synergistically with chemotherapy in lung cancer cell lines and primary cultures freshly made from lung cancer patients. Cancer Res. 2000;60:1009–1013.
Ganly I, et al. A phase I study of ONYX-015, and E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6:798–806.
Nemunaitis J, et al. A Phase II trial of intratumoral injection of ONYX-015 in-patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–298.
Nemunaitis J, et al. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, and E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60:6359–6366.
Khuri F, et al. A controlled trial of intratumoral ONYX-015, a selectively replicating adenovirus, in combination with cisplatin and 5-FU in patients with recurrent head and neck cancer. Nature Med. 2000;6:879–885.
Nemunaitis J, et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Therapy. 2001;8:746–759.
Barker DD, Berk AJ . Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral injection and DNA transfection. Virology. 1997;156:107–121.
Maron DJ, Hiroomi T, Moscioni AD, et al. Infra arterial delivery of a recombinant adenovirus does not increase gene transfer to tumor cells in a rat model of metastatic colorectal carcinoma. Mol Ther. 2001;4:29–35.
Li E, Stupack D, Bokoch GM, et al. Adenovirus endocytosis requires actin cytoskeleton organization mediated by Rho family GTPases. J Virol. 1998;72:8806–8812.
Wolff G, et al. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J Virol. 1997;71:624–629.
Biewenga J, et al. Macrophage depletion in the rat after intraperitoneal administration of liposome-encapsulated clodronate: depletion kinetics and accelerated repopulation of peritoneal and omental macrophages by administration of Fruend's adjuvant. Cell Tissue Res. 1995;280:189–196.
McCuskey RS, McCuskey PA, Urbaschek R, et al. Kupffer cell function in host defense. Rev Infect Dis. 1999;9:S616–S619.
Huitinga L, et al. Macrophages in T-cell line-medicated demyelination and chronic relapsing experimental autoimmune encephalomyelitis in Lewis rats. Clin Exp Immunol. 1995;10:344–351.
Laman JD, Kors N, van Rooijen N, et al. Mechanism of follicular trapping localization of immune complexes and cell remnants after elimination and repopulation of different spleen cell populations. Immunology. 1990;71:57–62.
Pinto AJ, Stewart D, van Rooijen N, et al. Selective depletion of liver and splenic macrophages using liposomes encapsulating the drug dichloromethylene diphosphonate: effects on antimicrobial resistance. J Leukoc Biol. 1991;49:579–586.
Qiam Q, Jutila A, van Rooijen, et al. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidates. J Immunol. 1994;152:5000–5008.
Tschaikowsky D, Brain JD . Effects of liposome-encapsulated dichloromethylene diphosphonate on macrophage function and endotoxin-induced mortality. Biochem Biophys Acta. 1994;1222:323–330.
Van Rooijen N . The liposome-mediated macrophage ‘suicide’ technique. J Immunol Meth. 1989;124:1–6.
Van Rooijen N, Kors N, Kraal G . Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J Leukoc Biol. 1989;45:97–104.
Van Rooijen N, Kors N, vd Ende M, et al.. Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome-encapsulated dichloromethylene diphosphonate. Cell tissue Res. 1990;260:215–222.
Van Rooijen N, Sanders A . Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Meth. 1994;174:83–93.
Van Rooijen N, Sanders A . Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology. 1996;23:1239–1243.
Vreden SG, et al. Kupffer cell elimination enhances development of liver schizonts of Plasmodium berghei in rats. Infect Immun. 1993;61:1936–1939.
Heideman DA, Snijders PJ, Craanen ME, et al. Selective gene delivery toward gastric and esophageal adenocarcinoma cells via EpCAM-targeted adenoviral vectors. Cancer Gene Therapy. 2001;8:342–351.
Seidman M, Hogan S, Wendland R, et al. Variation in adenovirus receptor expression and adenovirus vector-mediated transgene expression at defined stages of the cell cycle. Mol Ther. 2001;4:13–21.
Jordan MA, Toso RJ, Thrower D, et al. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci USA. 1993;90:9552–9556.
Long BH, Fairchild CR . Paclitaxel inhibits progression of mitotic cells to G1 phase by interference with spindle formation without affecting other microtubule functions during anaphase and telephase. Cancer Res. 1994;54:4355–4361.
Kay MA, Holterman AX, Meusel L, et al. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA41g administration. Nat Genet. 1995;11:191–197.
Crystal RG . Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet. 1994;8:42–51.
Yang Y, Li Q, Ertl HC, Wilson JM, et al. Cellular and tumoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenovirus. J Virol. 1995;69:2004–2015.
Yang Y, Greenough K, Wilson JM . Transient immune blockade prevents formation of neutralizing antibody to recombinant adenovirus and allows repeated gene transfer to mouse liver. Gene Therapy. 1996;3:412–420.
Dai Y . Cellular and tumoral immune response to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405.
Kass-Eisler A . The impact of development stage, route of administration and the immune system on adenovirus-mediated gene transfer. Gene Therapy. 1994;1:395–402.
Yang Y, Trinchieri G, Wilson JM . Recombinant IL-12 prevents formation of blocking IfA antibodies to recombinant adenovirus and allows repeated gene therapy to mouse lung. Nat Med. 1995;1:890–893.
Kolls JK, et al. Use of transient CD4 lymphocyte depletion to prolong transgene expression of E1-deleted adenoviral vectors. Hum Gene Ther. 1996;7:489–497.
Sawchuk SJ, et al. Anti-T-cell receptor monoclonal antibody prolongs transgene expression following adenovirus-mediated in vivo gene transfer to mouse synovium. Hum Gene Ther. 1996;7:499–506.
Bouvet M, et al. Suppression of the immune response to an adenovirus vector and enhancement of intratumoral transgene expression by low-dose etoposide. Gene Therapy. 1998;5:189–195.
Fang B . Gene therapy for hemophilia B: host immunosuppression prolongs the therapeutic effect of adenovirus-mediated factor IX expression. Hum Gene Ther. 1995;6:1039–1044.
Jooss K, Ertl HC, Wilson JM . Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J Virol. 1998;72:2945–2954.
Engelhardt JF, Ye X, Doranz B, et al. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196–6200.
Vilquin JT . FK506 immunosuppression to control the immune reactions triggered by first-generation adenovirus-mediated gene transfer. Hum Gene Ther. 1995;6:1391–1401.
Lochmuller H, et al. Immunosuppression by FK506 markedly prolongs expression of adenovirus-delivered transgene in skeletal muscles of adult dystrophic (mdx) mice. Biochem Biophys Res Commun. 1995;213:569–574.
Kass-Eisler A, Leinwand L, Gall J, Bloom B, Falck-Pedersen E . Circumventing the immune response to adenovirus-mediated gene therapy. Gene Ther. 1996;3:154–162.
Wickham TJ, Mathias P, Cheresh DA, Nemerow GR . Integrins and promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319.
Kjellen L, Pereira HG . Role of adenovirus antigens in the induction of virus neutralizing antibody. J Gen Virol. 1968;2:177–185.
Wohlfart C . Neutralization of adenoviruses: kinetics, stoichiometry and mechanisms. J Virol. 1988;62:2321–2328.
Greber UF, Willetts M, Webster P, Helenius A . Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486.
Bai M, Campisi L, Freimuth P . Vitronectin receptor antibodies inhibit infection of HeLa and A549 cells by adenovirus type 12 but not by adenovirus type 2. J Virol. 1994;68:5925–5932.
Yang Y, Xiang H, Ertl J, Wilson JM . Upregulation of Class I major histocompatibility complex antigens by γ-IFN is necessary for T-cell mediated elimination of recombinant adenovirus-injected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261.
Shenk T . Adenoviridae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM, eds. Fields Virology. Philadelphia, PA: Lippincott-Raven; 1996:2111.
Yang Y, Ertl HC, Wilson JM . MHC Class I-restricted cytotoxic T-lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442.
Zsengeller ZK, Wert SE, Hull WM, et al. Persistence of replication-deficient adenovirus-mediated gene transfer in lung of immune-deficient (nu/nu) mice. Hum Gene Ther. 1995;6:457–467.
Amena R, Van Tsai, Ann Goudreau, et al. Specific depletion of human anti-adenovirus antibodies facilitates transduction in an in vivo model for systematic gene therapy. Mol Ther. 2001:01:15-25-0016.
Lorence RM, Roberts MS, Groene WS, et al. Replication-competent, oncolytic Newcastle disease virus for cancer therapy. In: Driever PH, Roabkin SD, eds. Replication-Competent Viruses for Cancer Therapy. Basel, Switzerland: Karger, In press.
Wold WS, Hermiston TW, Tollefson AE . Adenovirus proteins that subvent host defenses. Trends Microbiol. 1994;2:437–443.
Wold WS, Tollefson AE, Hermiston TW . E3 transcription unit of adenovirus. Curr Top Microbiol Immunol. 1995;199:237–274.
Horwitz MS, Tufariello J, Grunhaus A . Model system for studying the effects of adenovirus E3 gene on virulence in vivo. Curr Top Microbiol Immunol. 1995;199:195–211.
Lee MG, Abina MA, Haddada H, et al. The constitutive expression of the immunomodulatory gp 19k protein in E1-E3 adenoviral vectors strongly reduces the host cytotoxic T-cell response against the vector. Gene Therapy. 1995;2:256–262.
Bett AJ, Haddara W, Prevec L, et al. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA. 1994;91:8802–8806.
Iian Y . Insertion of the adenoviral E3 region into a recombinant viral vector prevents antiviral tumoral and cellular immune responses and permits long-term gene expression. Proc Natl Acad Sci USA. 1997;94:2587–2592.
O’Neil WK . Toxicological comparison of E2A-deleted and first generation adenoviral vectors expressing alpha-1-antitrypsin after systemic delivery. Hum Gene Ther. 1998;9:1587–1598.
Elkon KB . Tumor necrosis factor α plays a central role in immune-mediated clearance of adenoviral vectors. Immunology. 1997;94:9814–9819.
Benihoud K . Efficient: repeated adenovirus-mediated gene transfer in mice lacking both tumor necrosis factor alpha and lymphotoxin α. J Virol. 1998;72:9514–9525.
Alcami A, Koszinowski UH . Viral mechanisms of immune evasion. Immunol Today. 2000;21:447–455.
Nash P, Barret JX, Cao S, et al. Immunomodulation by viruses: the myxoma virus story. Immunol Rev. 1999;168:103–120.
Smith GL, Symons JA, Khanna A, et al. Vaccinia virus immune evasion. Immunol Rev. 1997;159:137–154.
Smith VP, Bryant NA, Alcami A . Ectromelia, vaccinia and cowpox viruses encode secreted interleukin-18-binding proteins. J Gen Virol. 2000;81:1223–1230.
Spriggs M . One step ahead the game: viral immunomodulatory molecules. Annu Rev Immunol. 1996;14:101–130.
Saraiva M, Alcami A . CrmE, a novel soluble tumor necrosis factor receptor encoded by poxviruses. J Virol. 2001;75:226–233.
Mahr JA, Gooding LR . Immune evasion by adenoviruses. Immunol Rev. 1999;168:121–130.
Howar ST, Chan US, Smith GL . Vaccinia virus homologues of the Shope fibroma virus inverted terminal repeat proteins and a discontinuous ORF related to the tumor necrosis factor receptor family. Virology. 1991;180:633–647.
Smith CA, Davis T, Wignal JM, et al. T2 open reading frame from Shope fibroma virus encodes a soluble form of the TNF receptor. Biochem Biophys Res Commun. 1991;176:335–342.
Upton C, Macen JL, Schreiber M, et al. Myxoma virus expresses a secreted protein with homology to the tumor necrosis factor receptor gene family that contributes to viral virulence. Virology. 1991;184:370–382.
Pecora A, Rizvi N, Cohen G . A phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J Clin Oncol. 2002;20:2251–2266.
Hawkins LK, Johnson L, Bauzon M, et al. Gene delivery from the E3 region of replicating human adenovirus: evaluation of the 6.7 k/gp19K region. Gene Therapy. 2001;8:1123–1131.
Freytag S, Rogulski K, Paielli D, et al. A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene and radiotherapy. Hum Gene Ther. 1998;9:1323–1333.
Johnson L, Shen A, Boyle L, et al. Selectively replicating adenovirus targeting deregulated EZF activity are potent, systemic antitumor agents. Cancer Cell. 2002;1:325–337.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nemunaitis, J., Cunningham, C., Tong, A. et al. Pilot trial of intravenous infusion of a replication-selective adenovirus (ONYX-015) in combination with chemotherapy or IL-2 treatment in refractory cancer patients. Cancer Gene Ther 10, 341–352 (2003). https://doi.org/10.1038/sj.cgt.7700585
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700585
Keywords
This article is cited by
-
Ki67 targeted strategies for cancer therapy
Clinical and Translational Oncology (2018)
-
Oncolytic immunotherapy: unlocking the potential of viruses to help target cancer
Cancer Immunology, Immunotherapy (2017)
-
Oncoapoptotic Markers in Oral Cancer: Prognostics and Therapeutic Perspective
Molecular Diagnosis & Therapy (2014)
-
Engineered retroviral virus-like particles for receptor targeting
Archives of Virology (2014)
-
Clinical development directions in oncolytic viral therapy
Cancer Gene Therapy (2011)