Abstract
A well-known example of a positive association between the level of genetic variation and fitness in endangered species is the studies in Gila topminnow. The work of Vrijenhoek and his colleagues showed lower values for four fitness correlates in laboratory-raised fish from a population that was monomorphic for all 25 allozyme loci examined (Monkey Spring) than for fish from a population that was heterozygous for two of the allozyme loci (Sharp Spring). Here, bilateral asymmetry in wild-caught fish from these sites is examined to determine if the same environmental stressor (or one with similar effects) was present in natural populations of Gila topminnows. There were no differences for all three traits, lateral-line scales, pectoral-fin rays and pelvic-fin rays, previously found to be significantly different between Monkey Spring and Sharp Spring. This, coupled with our earlier finding that fish raised in our laboratory (where there is low mortality) had low bilateral asymmetry, supports the hypothesis that some unknown, and perhaps unnatural, environmental factor in the Vrijenhoek laboratory was responsible for the differences observed in bilateral asymmetry between Monkey Spring and Sharp Spring.
Similar content being viewed by others
Introduction
A commonly cited example of a positive association between genetic variation and fitness in endangered species is the research on Gila topminnows (Poeciliopsis o. occidentalis) (Vrijenhoek et al., 1985; Quattro & Vrijenhoek, 1989; Vrijenhoek, 1996). They found that Monkey Spring (MS) fish were monomorphic for 25 allozyme loci, whereas Sharp Spring (SS) fish were polymorphic for two of these loci. Laboratory-raised fish from MS had lower survival, growth rate and fecundity and more bilateral asymmetry than SS fish, suggesting a positive correlation between genetic variation and fitness.
Sheffer et al. (1997) detected no significant differences in bilateral asymmetry and survival for laboratory-raised fish from these sites. Further, higher growth rates were observed for MS than for SS (the opposite of that observed by Quattro & Vrijenhoek, 1989), and fecundity differences that appear to be environmentally induced (Sheffer et al., 1997). In addition, recent mtDNA analysis shows no difference between these sites (Quattro et al., 1996), and variation at a major histocompatibility complex (MHC) locus shows variation in MS fish as well as in SS fish (Hedrick & Parker, 1998). In other words, further investigation has not confirmed the earlier studies for either the measures of fitness or the amount of genetic variation.
It appears that the high mortality observed by Quattro & Vrijenhoek (1989) in all fish and the high asymmetry observed in MS fish may be the result of some unknown laboratory environmental stress (Sheffer et al., 1997). As a result, bilateral asymmetry in wild-caught Gila topminnows is examined for the three traits found to be significantly different between locations by Quattro & Vrijenhoek (1989) in an effort to determine if natural environmental conditions include stressors with effects similar to those that caused the reported differences in the bilateral asymmetry in laboratory-raised fish in the study of Quattro & Vrijenhoek (1989).
Materials and methods
The Gila topminnow (Poeciliopsis o. occidentalis) is a small, live-bearing, federally and state-listed endangered fish, once abundant throughout the lower Gila River drainage of Arizona (Hubbs & Miller, 1941). The natural populations are now limited to at most nine isolated locations, primarily in springs and stream headwaters (e.g. Sheffer et al., 1997). The fish examined here were wild-caught individuals from MS and SS, both isolated sites in south-eastern Arizona. MS is a thermally and chemically stable tributary to Sonoita Creek but isolated from that stream by a 10-m-high travertine barrier. SS is a thermally fluctuating, spring-fed tributary to the upper Santa Cruz River. The distance between the two sites by water, when water is present (long reaches are generally dry), is approximately 100 km.
In summer 1993, under U.S. Fish and Wildlife Endangered Species Subpermit Prt-676811, which allowed the capture of 20 Gila topminnows per site, 20 fish were obtained from MS. In addition, 13 fish were obtained from SS stock at nearby Heron Spring (the numbers of Gila topminnows in most areas of SS are quite low because it is highly infested with the introduced mosquito fish, Gambusia affinis). These fish were used to establish captive stocks at Arizona State University but only 13 from MS and 10 from SS were ultimately preserved and thus available for our present analysis. Consequently, to increase our sample size of wild-caught fish, 25 MS fish wild-caught in 1964 and 22 SS fish wild-caught in 1979, obtained from collections at the Arizona State University Museum, were also examined.
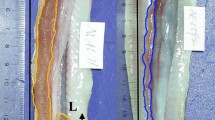
Before examination, the fish obtained in 1993 were fixed in 10 per cent formalin and stored in 70 per cent ethanol (the museum samples were treated in the same manner). For all samples, lateral-line scale counts on the right and left sides were first taken (top corner operculum to peduncle along lateral line; Hubbs & Lagler, 1964). The fish were then cleaned and stained, and right and left fin-ray counts were taken on the pectoral and pelvic fins. Counts for all three traits on all fish were made by C.S.
A variety of indices have been proposed for the measurement of bilateral asymmetry (e.g. Palmer & Strobeck, 1986; Palmer, 1994). For this study, we used the index referred to as fluctuating asymmetry:

where L indicates the count on the left side, R indicates the count on the right side of the same individual and N is the number of individuals in the sample. This measure provides good statistical power when sample sizes or differences between samples are small (Palmer, 1994). Differences in bilateral asymmetry levels between the populations and the level of significance within a population were tested using two-tailed t-tests (Zar, 1984). Anti-symmetry results in a platykurtic distribution, i.e. negative kurtosis (Palmer, 1994), and antisymmetry was tested for by calculating distributional statistics for both locations and all traits.
Results
First, it was determined if the wild-caught samples obtained at different times for each location were homogeneous. The mean trait size was homogeneous over time within location with an average difference per trait of only 2.8 per cent. Using a two-tailed t-test of FA values to determine temporal homogeneity of the three traits within the two different sites, the six probabilities ranged from 0.21 to 0.99 with a mean of 0.62. These results indicate no heterogeneity of FA within site over time for all three traits and, as a result, the MS samples from 1964 and 1993 and the SS samples from 1979 and 1993 were combined in the following analysis.
The bilateral asymmetry values for the wild-caught MS and SS samples are given for the three traits under (a) of Table 1 (the mean numbers per side of lateral-line scales, pectoral-fin rays and pelvic-fin rays, averaged over all fish, were 29.3, 14.2 and 5.8, respectively). No significant difference between populations was found for bilateral asymmetry in any of the three traits. The largest difference was for pelvic-fin rays, in which all MS fish were completely symmetrical and SS fish had a FA value of 0.09 (three out of 32 fish had a left–right difference of one fin ray). For pectoral- and pelvic-fin rays, levels of bilateral asymmetry of both populations were not significantly different from zero. Bilateral asymmetry for lateral-line scales was highly significantly different from zero (P<0.005 for both the MS and SS samples). For lateral-line scales, directional asymmetry and bias in left–right differences (Palmer & Strobeck, 1986) were tested for, but the mean left–right difference was not significantly different from zero. Overall, 68 per cent of the sampled topminnows (69 per cent for MS and 67 per cent for SS) were symmetrical for all three traits. There was no evidence of antisymmetry; in fact, all kurtosis values were positive.
Discussion
The present results are similar to our previous findings for laboratory-raised offspring of wild-caught females from MS and SS in 1994 [Sheffer et al., 1997; summarized under (b) in Table 1 ]. In that study, there were also no differences in FA values between MS and SS fish for all three traits, and all FA values were not significantly different from zero, including lateral-line scales. On the other hand, under (c) in Table 1 are given the bilateral asymmetry values for the laboratory-raised fish from the study of Quattro & Vrijenhoek (1989). Unlike our results, they found higher asymmetry for all three traits for laboratory-raised fish from MS than from SS, and the differences between sites are statistically significant (the comparison for pelvic-fin rays is only statistically different when their data from the related subspecies, P. o. sonoriensis, are included; Sheffer et al., 1997). The value of bilateral asymmetry they observed was statistically different from zero for MS for all three traits and not statistically different from zero for SS for all three traits. The numbers of fish in our wild-caught samples were similar to those in the previous studies, so that there was sufficient statistical power to detect bilateral asymmetry or differences in asymmetry, if either were present.
Comparison of the present results from wild-caught fish with results from previous studies of laboratory-reared fish raises several issues. The wild-caught fish used in the present study were adults. If bilateral asymmetry has fitness consequences for wild Gila topminnows, it is possible that more asymmetrical juveniles would be selected against and eliminated from the population, making it possible that less symmetrical fish have died and may not be present in our wild-caught samples of adults. However, the laboratory-raised fish examined by Sheffer et al. (1997), which were born from wild-caught mothers less than 14 days after they were brought into captivity, had very low asymmetry. There was also very little mortality (7 per cent and 4 per cent in the MS and SS samples, respectively, over 12 weeks) in these fish before they were measured. In other words, these fish should provide a reasonable baseline value of asymmetry for newborn fish because, with such a low level of mortality, it is highly unlikely that asymmetry at birth could be significantly higher than the low asymmetry observed at 12 weeks.
In our study of laboratory-reared fish (Sheffer et al., 1997), mortality was only 5.5 per cent over 12 weeks for the combined MS and SS samples, whereas in the study of Quattro & Vrijenhoek (1989) mortality was about 50 per cent over 12 weeks (55 per cent in MS and 44 per cent in SS). The higher bilateral asymmetry and the differences between MS and SS observed by Quattro & Vrijenhoek (1989) may be caused by the same factors that resulted in higher mortality in their study. In all of our wild-caught and laboratory-raised fish in Table 1, all traits were either symmetrical or differed by a left–right difference of one. This was also generally true for the data of Quattro & Vrijenhoek (1989) (R. Vrijenhoek, pers. comm.), except that two MS individuals differed by two and three pelvic-fin rays, respectively, and two SS individuals both differed by two pelvic-fin rays. A difference of two or three pelvic-fin rays results in approximately a 30 per cent left–right difference and suggests that the laboratory conditions that caused high mortality also caused extreme asymmetry, even in some of the fish that survived for 12 weeks.
It appears that some unknown, and perhaps unnatural, environmental stress was present under the laboratory conditions of Quattro & Vrijenhoek (1989), which resulted in high mortality in both MS and SS fish and more bilateral asymmetry in MS (see Sheffer et al., 1997 for further discussion). It is possible that this difference in asymmetry between sites may indicate some unknown genetic differences in sensitivity to environmental stress, but because the potential environmental stressor is unknown, it is difficult to make strong inferences from these data (see Sheffer et al., 1997). Further, because differential levels of bilateral asymmetry between wild-caught MS and SS fish were not observed, it does not appear that this environmental stress (or one with similar effects) is important in the natural environment.
Although it is appealing to have simple answers to genetic questions related to conservation, we must be circumspect in our recommendations based on genetics. In the case of the endangered Gila topminnow, it appears that the phenotypic differences observed between locations for fitness correlates were not genetic and were the result of laboratory environmental conditions for survival, growth rate and bilateral asymmetry and natural environmental differences for fecundity. The association of these presumed fitness values with allozyme heterozygosity in two populations of Gila topminnows appears to be largely coincidental and should not be used for a general prescription for conservation recommendations. However, it should be noted that, based on the recommendations of Quattro & Vrijenhoek (1989), the captive stock from MS was replaced with one from SS, and subsequent reintroductions have used only SS fish (Sheffer et al., 1997).
Finally, it is important to publish results that do not give statistically significant differences. If they are not reported, then conclusions that may be wrong or at least oversimplistic become legend in the literature. In particular, it is not clear if or when the level of bilateral asymmetry is correlated with levels of genetic variation, but it is important to note that several recent studies suggest that a positive correlation is not a universal phenomenon (Clarke et al., 1992; Fowler & Whitlock, 1994; for perspectives, see Clarke, 1995; Palmer, 1996).
References
Clarke, G. M. (1995). Relationships between developmental stability and fitness: application for conservation biology. Conserv Biol, 9: 18–24.
Clarke, G. M., Oldroyd, B. P. and Hunt, P. (1992). The genetic basis of developmental stability in Apis mellifera: heterozygosity vs. genic balance. Evolution, 46: 753–762.
Fowler, K. and Whitlock, M. C. (1994). Fluctuating asymmetry does not increase with moderate inbreeding in Drosophila melanogaster. Heredity, 73: 373–376.
Hedrick, P. W. and Parker, K. M. (1998). MHC variation in the endangered Gila topminnow. Evolution. (in press).
Hubbs, C. L. and Lagler, K. F. (1964). Fishes of the Great Lakes Region. University Michigan Press, Ann Arbor.
Hubbs, C. L. and Miller, R. R. (1941). Studies of the order Cyprinodontes, XVII. Genera and species of the Colorado River system. Occ Pap Mus Zool, Univ Mich, 433: 1–9.
Palmer, A. R. (1994). Fluctuating asymmetry analyses: a primer. In: Markow, T. A. (ed.) Developmental Instability: Its Origins and Evolutionary Implications, pp. 335–364. Kluwer, Boston, MA.
Palmer, A. R. (1996). Waltzing with asymmetry. Bioscience, 46: 518–532.
Palmer, A. R. and Strobeck, C. (1986). Fluctuating asymmetry: measurement, analysis, patterns. Ann Rev Ecol Syst, 17: 391–421.
Quattro, J. M. and Vrijenhoek, R. C. (1989). Fitness differences among remnant populations of the endangered Sonoran Topminnow. Science, 245: 976–978.
Quattro, J. M., Leberg, P. L., Douglas, M. E. and Vrijenhoek, R. C. (1996). Molecular evidence for a unique evolutionary lineage of endangered Sonoran desert fish (genus Poeciliopsis). Conserv Biol, 10: 128–135.
Sheffer, R. J., Hedrick, P. W., Minckley, W. L. and Velasco, A. (1997). Fitness in the endangered Gila topminnow. Conserv Biol, 11: 162–171.
Vrijenhoek, R. C. (1996). Conservation genetics of North American fishes. In: Avise, J. C. and Hamrick, J. L. (eds) Conservation Genetics: Case Histories from Nature, pp. 367–397. Chapman and Hall, New York.
Vrijenhoek, R. C., Douglas, M. E. and Meffe, G. K. (1985). Conservation genetics of endangered fish populations in Arizona. Science, 229: 400–402.
Zar, J. H. (1984). Biostatistical Analysis. Prentice Hall, Englewood Cliffs, NJ.
Acknowledgements
This research was supported financially in part by the National Science Foundation (P.W.H. and R.J.S.) and the National Institutes of Health MARC programme (K.S.). W. L. Minckley and anonymous reviewers made important comments on various drafts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheffer, R., Hedrick, P. & Shirley, C. No bilateral asymmetry in wild-caught, endangered Poeciliopsis o. occidentalis(Gila topminnows). Heredity 80, 214–217 (1998). https://doi.org/10.1046/j.1365-2540.1998.00299.x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1046/j.1365-2540.1998.00299.x