Abstract
In the Hymenoptera, males develop as haploids from unfertilized eggs and females develop as diploids from fertilized eggs. In species with complementary sex determination (CSD), however, diploid males develop from zygotes that are homozygous at a highly polymorphic sex locus or loci. We investigated mating behavior and reproduction of diploid males of the parasitoid wasp Cotesia vestalis (C. plutellae), for which we recently demonstrated CSD. We show that the behavior of diploid males of C. vestalis is similar to that of haploid males, when measured as the proportion of males that display wing fanning, and the proportion of males that mount a female. Approximately 29% of diploid males sired daughters, showing their ability to produce viable sperm that can fertilize eggs. Females mated to diploid males produced all-male offspring more frequently (71%) than females mated to haploid males (27%). Daughter-producing females that had mated to diploid males produced more male-biased sex ratios than females mated to haploid males. All daughters of diploid males were triploid and sterile. Three triploid sons were also found among the offspring of diploid males. It has been suggested that this scenario, that is, diploid males mating with females and constraining them to the production of haploid sons, has a large negative impact on population growth rate and secondary sex ratio. Selection for adaptations to reduce diploid male production in natural populations is therefore likely to be strong. We discuss different scenarios that may reduce the sex determination load in C. vestalis.
Similar content being viewed by others
Introduction
In the insect order Hymenoptera, males typically develop from unfertilized haploid eggs, and females from fertilized diploid eggs. However, diploid males can occur as a result of complementary sex determination (CSD) in which diploid offspring develop as males when they are homozygous at a sex locus, and as females when they are heterozygous at this locus (for example, Cook and Crozier, 1995; Beye et al., 2003; Wu et al., 2003; van Wilgenburg et al., 2006). The frequency of diploid males in a population depends on the level of inbreeding and on the diversity of sex alleles. Sex allele diversity has been estimated for a few species; examples are 15–86 sex alleles in the fire ant Solenopsis invicta (Ross and Fletcher, 1985; Ross et al., 1993), 19 in the honeybee Apis mellifera (Adams et al., 1977; see also Hasselmann and Beye, 2004) and 9–20 in the parasitoid wasp Habrobracon hebetor (Whiting, 1943; Heimpel et al., 1999; Antolin et al., 2003). Because sex allele diversity is high and species with CSD may avoid inbreeding (Ode et al., 1995), diploid male frequencies in the field are generally low (for example, Owen and Packer, 1994; Takahashi et al., 2001). Yet to date, diploid males have been reported in more than 60 species (van Wilgenburg et al., 2006).
Diploid males can differ from haploid males in several characteristics. In some species, diploid males are larger than haploid males, for example in the sawflies Athalia rosae and Neodiprion nigroscutum (Smith and Wallace, 1971; Naito and Suzuki, 1991). In other species, diploid males may be smaller than haploid males, but this has only been reported for the bumblebee Bombus terrestris (Duchateau and Marien, 1995). Diploid males may have lower survival rates than haploid males and females. For example, viability of diploid males is generally less than 5% in the parasitoid wasp H. hebetor, and most of them die during the egg stage (Whiting, 1943; Petters and Mettus, 1980). However, diploid male survival can vary widely, even between closely related species, and is much higher in H. brevicornis, H. serinopae and H. sp. near hebetor (Speicher and Speicher, 1940; Clark et al., 1963; Holloway et al., 1999). In the honeybee A. mellifera, workers actively kill and remove diploid males as early larvae (Woyke, 1963).
When diploid males survive, three important questions arise: Do diploid males mate with females? If so, what are the consequences of such matings? And, what is the relative reproductive success of diploid males compared to haploid males? Reproduction by diploid males has been studied in a range of hymenopteran species, including parasitoid wasps, ants and bumblebees. The results of these studies can be classified into three groups: (1) diploid males do not mate with females (for example, the conifer sawfly N. nigroscutum: Smith and Wallace, 1971); (2) diploid males mate with females but are sterile, therefore restricting females to the production of haploid offspring only (for example, H. sp. near hebetor: Holloway et al., 1999); (3) diploid males mate with females and produce sterile triploid daughters and sometimes sons (for example, Polistes spp: Liebert et al., 2005). In all these cases, diploid males do not produce any fertile offspring and it has thus generally been accepted that diploid males are a reproductive dead end in species with CSD. However, Cowan and Stahlhut (2004) recently showed that diploid males of the vespid wasp Euodynerus foraminatus are fertile. They produce diploid female offspring when crossed with an unrelated diploid female, possibly because the reduction division during spermatogenesis is restored. Their study thus added a fourth possible outcome of interactions between diploid males and females, and a cautionary note about generalizing the reproductive fate of diploid males without doing experiments. Stouthamer et al. (1992) used simulations to show that the negative impact of diploid male production on sex ratio and population growth rate is the strongest when diploid males survive and mate, constraining females to produce only haploid sons. The population-level consequences of CSD thus depend on the outcome of interactions between diploid males and females, warranting studies on these interactions in any species with CSD.
The genus Cotesia (Braconidae: Microgastrinae) is a valuable system to study the relationships between sex determination, life history traits and behavior. Interestingly, this large genus of parasitoids comprises species with and species without CSD. Niyibigira et al. (2004a) demonstrated the absence of CSD in the gregarious stemborer parasitoid C. flavipes, and sex ratio data from field populations suggest CSD is very unlikely in C. sesamiae (Niyibigira et al., 2004b). However, CSD was recently demonstrated in C. glomerata (Gu and Dorn, 2003; Zhou et al., 2006), and it has been suggested to operate in C. rubecula (personal communication by Steiner in Stouthamer et al., 1992; de Boer unpublished). In addition, we have also demonstrated CSD in C. vestalis, a solitary endoparasitoid of diamond back moth larvae (de Boer et al., 2007). Diploid male survival in C. glomerata is estimated to be rather low (around 26%), whereas it appears to be relatively high in C. vestalis (approximately 70%). To date, the behavior and reproductive function of diploid males have not been investigated in any Cotesia species. Here we study the mating behavior of diploid males of C. vestalis and describe their offspring. The species of Cotesia we study here has commonly been called Cotesia (or formerly Apanteles) plutellae Kurdjumov in the literature, but now should be referred to as C. vestalis (Haliday) (Shaw, 2003).
Methods
Source material
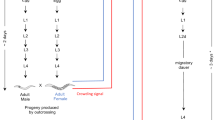
To study the behavior of and reproduction by diploid C. vestalis males, we used offspring from iso-female lines that were known to produce both haploid and diploid males (de Boer et al., in preparation). These lines were set up to determine the number of loci on which CSD is based in C. vestalis. In short, iso-female lines were started with a mother–son cross (see de Boer et al., 2007), using female offspring of outcrosses between two unrelated colonies of C. vestalis: colony A was started with about 200 adult wasps obtained from Biofac Crop Care (Mathis, TX, USA) and colony B was started with about 90 adult wasps from Benin (supplied by Dr D Bordat, CIRAD, Montpellier-sur-Lez, France). Iso-female lines were maintained on diamond back moth larvae, Plutella xylostella L., for 6–8 generations of sibmating to ensure the production of diploid males before they were used in our experiment. In these generations, most lines produced between 30 and 50% diploid males from fertilized offspring (de Boer et al., in preparation).
Reproduction by diploid males
Newly emerged female offspring from the lines described above were paired with a brother in a plastic tube containing a piece of host-damaged cabbage leaf and a small droplet of honey. Observations were made without knowledge of the male's ploidy, which was determined afterwards (see below). We observed behavior of the pair of wasps for 10 min, recording the display of wing fanning, mounting attempts (when males approached females but the female walked away) and the occurrence of successful mounting (defined by the male staying in contact with the female for at least 30 s).
After observations, we left all pairs together for 48 h at 23±1°C to allow mating if it had not yet occurred. Males were then frozen at −30°C for analysis of ploidy. The female was exposed to approximately 15–20 second and third instar diamondback moth larvae on cabbage in a plastic tube (2.5 cm diameter, 6.5 cm high) for 24 h. This was repeated once more for a total of 29–42 hosts for each female. Hosts were reared individually on artificial diet (modified from Shelton et al., 1991) in 1 oz clear cups in a growth chamber at 25±1°C until parasitoid cocoons or moth pupae developed, or hosts died. Cocoons were individually placed in microcentrifuge tubes. Emerging wasps were sexed and frozen for analysis of ploidy, with the exception of 12 daughters of diploid males (see below).
A total of 64 crosses was made, of which 33 and 29 were initiated with a haploid and a diploid male respectively, while the ploidy of two males could not be determined. One female crossed with a diploid male died before she was offered any hosts, so only behavioral data were recorded for this replicate.
After identification of their ploidy, a subset of 10 haploid and 10 diploid males was dissected to determine whether their internal reproductive organs were different. Wasps were dissected in water by pulling out the reproductive tract with an insect pin; the reproductive organs were then transferred to a microscope slide. Reproductive organs were measured under a compound microscope at × 100, and the presence of sperm was determined at × 1000. The presence of sperm in female spermathecae was not determined.
Statistical analyses
We used 2 × 2 contingency tables to analyze the effect of male ploidy on the occurrence of wing fanning, approaching and mounting, and on mating success (that is, pairs that produced female offspring; pairs without daughters were thought to have had no successful copulation). For the pairs that produced daughters, we statistically analyzed the effect of male ploidy on sex ratio with a logistic regression treating sex ratio as a binary response variable using PROC LOGISTIC in SAS (SAS Institute Inc., 2006). We analyzed the effect of male ploidy and whether daughters were produced on hosts by using a generalized logits model (PROC LOGISTIC, SAS Institute Inc., 2006) to compare the proportion of hosts that died, developed into moths, or developed into cocoons.
Reproduction by triploid females
Twelve virgin daughters of diploid males were exposed to 11–21 hosts each by placing them individually in a plastic tube with a piece of cabbage leaf and a small droplet of honey for 24 h. The hosts were reared as described above. Females were subsequently transferred to a new tube with host-damaged cabbage and a haploid male from an unrelated inbred line (males were known to be haploid because they were produced by a virgin diploid female). They were kept together for 48 h to allow mating. Females were then exposed to another set of 16–23 hosts for 24 h. Females were frozen for analysis of ploidy and for dissections to determine whether they contained any mature eggs. In this way, reproduction by virgin and mated triploid females was tested.
Statistical analysis
We analyzed the effect of triploid females on their hosts by using a generalized logits model (PROC LOGISTIC, SAS Institute Inc., 2006) to compare the proportion of hosts that died, developed into moths or developed into cocoons, with a set of control hosts. The controls consisted of hosts that were treated in the same way but were not exposed to a C. vestalis female. The two sets of hosts offered to each triploid female (before and after mating) were combined because we found no differences between the two sets in the number of offspring produced or the proportion of hosts that died (t-tests, P>0.05).
Flow cytometry
We used flow cytometry to identify the ploidy of C. vestalis wasps. Samples were prepared by pulverizing the head (without antennae) of an individual wasp in 0.5 ml ice-cold Galbraith buffer (21 mM MgCl2; 30 mM tri-sodium citrate dehydrate; 20 mM 3-[N-morpholino] propane sulfonic acid; 0.1% Triton X-100; 1 mg/l RNase A; Galbraith et al., 1983) in a Dounce tissue grinder (Kontes, Vineland, NJ, USA) by turning a B pestle 20 times. The homogenate was subsequently filtered through a cell strainer cap on a 5 ml polystyrene tube (Falcon, BD Biosciences, San Jose, CA, USA). Nuclei were stained with 20 μl propidium iodide (1.25 mg/ml) per sample. Flow cytometric analyses were performed on a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). An excitation wave length of 488 nm and a band pass filter of 585 nm were used to detect propidium iodide fluorescence. For each sample, 2500 nuclei were measured in an FL2-W/FL2-A gated region that contained haploid, diploid and tetraploid cells, using CELL Quest Pro. A threshold on FL2-A was used to exclude very small debris. We used flow cytometric DNA-histograms of known haploid males and diploid females as references to determine ploidy of unknown males and females (Figure 1).
We analyzed the ploidy of (1) all 64 males used as fathers in the behavior experiment, (2) all 32 daughters of diploid males and (3) 32 sons of females mated to diploid males.
Results
Behavior of diploid males
Wing fanning was observed in 19 out of 33 haploid males and 23 out of 29 diploid males (Figure 2) (2 × 2 contingency table, χ21=2.95, P=0.09). Male ploidy did not affect the number of males that approached a female (followed by the female walking away); this was observed in 8 out of 33 haploid males and in 12 out of 29 diploid males (2 × 2 contingency table, χ21=2.07, P=0.15). Diploid males were also observed mounting females as often as haploid males: seven out of 33 haploid males, and 12 out of 29 diploid males (2 × 2 contingency table, χ21=3.34, P=0.07). Diploid males thus appear to display normal mating behavior, and females appear to readily accept diploid males as mates.
Proportion of haploid (open, N=33) and diploid males (hatched bars, N=29) that displayed wing fanning, approached a female or mounted a female during a 10 min observation period, proportion of females mated to a haploid or diploid male that produced daughters and sex ratio of offspring of those females that produced at least one daughter (average±s.e.m.) are shown. Proportions of replicates for each parameter were analyzed with 2 × 2 contingency tables (NS P>0.05; *P<0.05); sex ratio was analyzed with logistic regression (*P<0.05).
Reproduction by diploid males
Even though mating behavior of diploid males was not different from that of haploid males, the proportion of replicates without daughters was significantly higher for diploid males: only 8 out of 28 replicates with a diploid male produced female offspring, while 24 out of 33 replicates with a haploid male produced daughters (Figure 2) (2 × 2 contingency table, χ21=11.8, P<0.001). Of those replicates for which mounting was observed during the first 10 min, 1 out of 7 involving a haploid male did not result in female offspring, while 6 out of 11 replicates involving a diploid male did not produce any daughters (2 × 2 contingency table, χ21=2.9, P=0.09).
Of the pairs producing daughters, the adult sex ratio was male-biased for replicates with haploid male fathers (69%) as well as for those with diploid male fathers (80%) (Figure 2). Females mated to diploid males were 1.76 times more likely to produce male offspring than females mated to haploid males (Wald χ21=6.61; P=0.01). However, the proportion of hosts that developed into moths, died or developed into parasitoid wasps was not significantly related to the ploidy of the father nor to whether or not daughters were produced (Table 1; P>0.05 in all cases).
Eight diploid males sired 32 daughters, of which 29 could be analyzed for ploidy. All daughters of diploid males proved to be triploid (Figure 1). We analyzed the ploidy of the male offspring from the three replicates that had the highest number of daughters. In total, 32 males were analyzed: 27 were haploid and 3 were triploid (2 samples failed to show clear peaks on the flow cytometric DNA-histograms and were recorded as unknown). All three triploid males were found in the offspring of one replicate.
The internal reproductive organs did not differ visibly between haploid and diploid males and testes length was the same for haploid (246.24±10.65 μm) and diploid males (243.39±9.04 μm). Sperm was observed in the testes of most haploid (8/10) and diploid males (9/10), including five diploid males that did not sire any daughters.
Reproduction by triploid females
Even though we observed triploid females stinging their hosts, they did not reproduce (Table 2). Only one cocoon developed from the hosts exposed to triploid females. This cocoon developed from a host exposed to a mated female, and it developed into a diploid male. Flow cytometry cannot reveal whether this male was biparental or uniparental. However, the proportion of parasitoid cocoons produced was not significantly different from that found in the set of control hosts, and so might have been caused by a low level of contamination of supposedly unparasitized hosts (Wald χ21=0.33; P=0.57). Hosts exposed to a triploid female were 7.9 times more likely to die than control hosts that had not been exposed to wasps (Wald χ21=67.22; P<0.001), suggesting that the females indeed stung some hosts, and possibly injected venom.
Dissections of the females showed that their ovaries developed normally and they contained mature eggs.
Discussion
Diploid males in C. vestalis appear to behave normally in interactions with females, displaying wing fanning and mounting females. Females also accept diploid males for mating. Similar findings have been reported in, for example, the parasitoid wasp Diadromus pulchellus (El Agoze et al., 1994) and the ant Lasius sakagamii (Yamauchi et al., 2001).
Even though diploid males courted and mounted females normally, only 29% of diploid males actually reproduced, in contrast to 73% for haploid males. In addition, females mated to diploid males produced a more male-biased sex ratio than those mated to haploid males. One possible explanation is that not all diploid males actually produce sperm. In several other species of Hymenoptera, male ploidy was found to affect the morphology of reproductive organs and/or sperm production. Tavares et al. (2003) showed that diploid male testes of the social bee Melipona quadrifasciata are significantly shorter than those of haploid males, but sperm production or reproduction by diploid males was not studied. Testes of diploid males are also smaller than those of haploid males in A. mellifera (Woyke, 1973, 1974) and B. terrestris (Duchateau and Marien, 1995). Hung et al. (1974) reported that in diploid males of Solenopsis invicta, development of the testicular lobes generally does not occur, and 98% of diploid males from a natural population were aspermic (Krieger et al., 1999). In contrast to these cases, the internal reproductive organs of diploid males of C. vestalis did not appear to differ from those of haploid males and sperm was observed in 9 out of 10 diploid males. Moreover, sperm was observed in five diploid males that did not sire any daughters. Potential explanations for the lack of reproduction by diploid males include a failure to transfer sperm to females during copulation, a failure of sperm to fertilize eggs or low viability of triploid zygotes (for example, El Agoze et al., 1994).
The triploid nature of the daughters of diploid males implies that diploid C. vestalis males produce diploid sperm. Diploid males of the related C. glomerata also produce diploid sperm (Zhou et al., 2006) but it has not been determined whether females of this species mate with diploid males and produce triploid offspring. With the exception of E. foraminatus (Cowan and Stahlhut, 2004), diploid males of all species that have been studied so far produce diploid sperm and triploid progeny, if any (for example, Hoshiba et al., 1981; Chauvin et al., 1987; Yamauchi et al., 2001).
Triploid females of C. vestalis did not reproduce, also confirming findings for other Hymenoptera, such as H. sp. near hebetor, and Polistes dominulus (Holloway et al., 1999; Liebert et al., 2004). The triploid females did not differ morphologically from diploid females and their ovaries appeared normal. We also observed them stinging hosts and we expect they injected eggs. It seems likely that the eggs they produced were unviable aneuploids. In contrast, triploid females of the parasitoid wasp Nasonia vitripennis produce a significant proportion of viable eggs that commonly develop into haploid or diploid males (Whiting, 1960). Recently, Beukeboom and Kamping (2006) showed that unmated triploid females of N. vitripennis also occasionally produce uniparental diploid females or gynandromorphs. Whether or not triploid females produce any viable eggs may be related to the haploid chromosome number, which is much lower in N. vitripennis (N=5) than in other hymenopterans studied so far, for example B. terrestris (N=18) or H. hebetor (N=10). A lower chromosome number may by chance result in a higher proportion of viable haploid or diploid eggs compared to inviable aneuploid eggs than a higher chromosome number. The haploid chromosome number of C. vestalis has not been determined, but the closely related C. glomerata has 10 chromosomes (Zhou et al., 2006).
Very few studies have reported triploid males among the offspring of diploid males crossed with related females. Under sl-CSD, triploid males are expected to occur as often as triploid females when parents share a sex allele. Although we believe that CSD in C. vestalis is based on more than one locus (de Boer et al., 2007), the wasps we used originated from lines that produced between 30 and 50% diploid males from fertilized eggs after inbreeding for 6–8 generations. We thus expected females mated to their diploid brothers to produce triploid sons and daughters in a 1:1 to 1:2.3 ratio. However, we only found three triploid males, compared to 21 females (ratio 1:7). Similar findings have been reported for the sawfly Athalia rosae ruficornis, where some females mated to diploid males produced triploid sons as well as daughters but others only produced triploid daughters (Naito and Suzuki, 1991). This may be explained by reduced survival of triploid males compared to triploid females, which has also been suggested for H. hebetor (Cook, 1993). To date, equal numbers of triploid males and females have only been reported for the bumblebee Bombus terrestris (Ayabe et al., 2004). We did not test the reproductive function of the triploid males, but we expect them to be sterile.
Progeny of diploid C. vestalis males are sterile triploid females, or triploid males. Diploid males are therefore an evolutionary dead end. According to model predictions, this scenario has the most negative effect on population growth rate and sex ratio (Stouthamer et al., 1992). We therefore expect selection to be strong on mechanisms or behaviors that reduce diploid male frequencies, that is, the genetic load, in natural populations. There are several scenarios that may reduce the genetic load associated with CSD in C. vestalis. First, we suggest that CSD in C. vestalis is based on more than one locus (de Boer et al., 2007; de Boer et al., in preparation). Under multiple locus-CSD, diploid males only develop from fertilized eggs that are homozygous at each of two or more sex loci, and the frequency of diploid males is significantly lower than under sl-CSD (Crozier, 1971). Second, the inbreeding frequency in the field may be low, which would result in a low frequency of diploid males, especially under ml-CSD. C. vestalis is a solitary parasitoid on a solitary host, which should make outbreeding likely in this species, but the actual mating structure has not been characterized. Inbreeding frequencies in the related C. glomerata that also exhibits CSD have been shown to be lower than expected for gregarious species (Gu and Dorn, 2003). The pattern of diploid male production in this species is largely consistent with sl-CSD (Zhou et al., 2006, 2007), but ml-CSD has not yet been ruled out (Gu and Dorn, 2003). In particular, the possibility that all but one locus in an ml-CSD system were fixed at the onset of these studies was not addressed in the studies on C. glomerata.
We showed that females accept diploid males as mates under laboratory conditions but the relative mating success of diploid and haploid males in the field is unknown. It is possible that diploid males achieve a smaller proportion of matings in the field than would be expected based on the relative occurrence of haploid and diploid males, for example through female choice, or reduced female searching or mating capacity of diploid males. Finally, after mating with a diploid male, females may remate with a haploid male. It would be important to determine whether this happens or not, and if it happens, whether haploid sperm has a competitive advantage over diploid sperm, or females can use it preferentially. Sperm precedence in double matings with one haploid and one diploid male has only been studied in one species with CSD, the ichneumonid parasitoid D. pulchellus (El Agoze et al., 1995). Females that are mated to a diploid male are restricted to the production of haploid male offspring in D. pulchellus because eggs that are fertilized with diploid sperm die (El Agoze et al., 1994). However, remating with a haploid male does not relieve females from this constraint because they will only use sperm of the first male they mated with.
In conclusion, our findings on the interactions between diploid males and females of C. vestalis confirm those of most previous studies on Hymenoptera with CSD: diploid males can mate but any offspring they get are sterile triploids. It thus appears that the findings of Cowan and Stahlhut (2004) of reproductively functional diploid males in E. foraminatus remain an exception.
References
Adams J, Rothman ED, Kerr WE, Paulino ZL (1977). Estimation of the number of sex alleles and queen matings from diploid male frequencies in a population of Apis mellifera. Genetics 86: 583–596.
Antolin MF, Ode PJ, Heimpel GE, O'Hara RB, Strand MR (2003). Population structure, mating system, and sex-determining allele diversity of the parasitoid wasp Habrobracon hebetor. Heredity 91: 373–381.
Ayabe T, Hoshiba H, Ono M (2004). Cytological evidence for triploid males and females in the bumblebee, Bombus terrestris. Chromosome Res 12: 215–223.
Beukeboom LW, Kamping A (2006). No patrigenes required for femaleness in the haplodiploid wasp Nasonia vitripennis. Genetics 172: 981–989.
Beye M, Hasselmann M, Fondrk MK, Page RE, Omholt SW (2003). The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell 114: 419–429.
Chauvin G, El Agoze M, Hamon C, Huignard J (1987). Ultrastructure des spermatozoïdes mâles haploïdes et diploïdes de Diadromus pulchellus Wesmeal (Hymenoptera: ichneumonidae). Int J Insect Morphol Embryol 17: 358–366.
Clark AM, Bertrand HA, Smith RE (1963). Life span differences between haploid and diploid males of Habrobracon serinopae after exposure as adults to X rays. Am Nat 97: 203–208.
Cook JM (1993). Sex determination in the Hymenoptera: a review of models and evidence. Heredity 71: 421–435.
Cook JM, Crozier RH (1995). Sex determination and population biology in the Hymenoptera. Trends Ecol Evol 10: 281–286.
Cowan DP, Stahlhut JK (2004). Functionally reproductive diploid and haploid males in an inbreeding hymenopteran with complementary sex determination. Proc Nat Acad Sci USA 101: 10374–10379.
Crozier RH (1971). Heterozygosity and sex determination in haplo-diploidy. Am Nat 105: 399–412.
de Boer JG, Ode PJ, Vet LEM, Whitfield J, Heimpel GE (2007). Complementary sex determination in the parasitoid wasp Cotesia vestalis (C plutellae). J Evol Biol 20: 340–348.
Duchateau MJ, Marien J (1995). Sexual biology of haploid and diploid males in the bumble bee Bombus terrestris. Insect Soc 42: 255–266.
El Agoze M, Drezen JM, Renault S, Periquet G (1994). Analysis of the reproductive potential of diploid males in the wasp Diadromus pulchellus (Hymenoptera, Ichneumonidae). Bull Entomol Res 84: 213–218.
El Agoze M, Poirie M, Periquet G (1995). Precedence of the first male sperm in successive matings in the Hymenoptera Diadromus pulchellus. Entomol Exp Appl 75: 251–255.
Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983). Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220: 1049–1051.
Gu HN, Dorn S (2003). Mating system and sex allocation in the gregarious parasitoid Cotesia glomerata. Anim Behav 66: 259–264.
Hasselmann M, Beye M (2004). Signatures of selection among sex-determining alleles of the honey bee. Proc Nat Acad Sci USA 101: 4888–4893.
Heimpel GE, Antolin MF, Strand MR (1999). Diversity of sex-determining alleles in Bracon hebetor. Heredity 82: 282–291.
Holloway AK, Heimpel GE, Strand MR, Antolin MF (1999). Survival of diploid males in Bracon sp. near hebetor (Hymenoptera: Braconidae). Ann Entomol Soc Am 92: 110–116.
Hoshiba H, Okada I, Kusanagi A (1981). The diploid drone of Apis cerana japonica and its chromosomes. J Apic Res 20: 143–147.
Hung ACF, Vinson SB, Summerlin JW (1974). Male sterility in the red imported fire ant, Solenopsis invicta. Ann Entomol Soc Am 67: 909–912.
Krieger MJB, Ross KG, Chang CWY, Keller L (1999). Frequency and origin of triploidy in the fire ant Solenopsis invicta. Heredity 82: 142–150.
Liebert AE, Johnson RN, Switz GT, Starks PT (2004). Triploid females and diploid males: underreported phenomena in Polistes wasps? Insect Soc 51: 205–211.
Liebert AE, Sumana A, Starks PT (2005). Diploid males and their triploid offspring in the paper wasp Polistes dominulus. Biol Lett 1: 200–203.
Naito T, Suzuki H (1991). Sex determination in the sawfly, Athalia rosae ruficornis (Hymenoptera): occurrence of triploid males. J Hered 82: 101–104.
Niyibigira EI, Overholt WA, Stouthamer P (2004a). Cotesia flavipes Cameron (Hymenoptera: Braconidae) does not exhibit complementary sex determination (ii) – Evidence from laboratory experiments. Appl Entomol Zool 39: 717–725.
Niyibigira EI, Overholt WA, Stouthamer R (2004b). Cotesia flavipes Cameron and Cotesia sesamiae (Cameron) (Hymenoptera: Braconidae) do not exhibit complementary sex determination: evidence from field populations. Appl Entomol Zool 39: 705–715.
Ode PJ, Antolin MF, Strand MR (1995). Brood-mate avoidance in the parasitic wasp Bracon hebetor Say. Anim Behav 49: 1239–1248.
Owen RE, Packer L (1994). Estimation of the proportion of diploid males in populations of Hymenoptera. Heredity 72: 219–227.
Petters RM, Mettus RV (1980). Decreased diploid male viability in the parasitic wasp, Bracon hebetor. J Hered 71: 353–356.
Ross KG, Fletcher DJC (1985). Genetic origin of male diploidy in the fire ant, Solenopsis invicta (Hymenoptera: Formicidae), and its evolutionary significance. Evolution 39: 888–903.
Ross KG, Vargo EL, Keller L, Trager JC (1993). Effect of a founder event on variation in the genetic sex-determining system of the fire ant Solenopsis invicta. Genetics 135: 843–854.
Shaw MR (2003). Revised synonymy in the genus Cotesia (Hymenoptera: Braconidae: Microgastrinae): the identity of Microgaster vestalis Haliday,1834, as a senior synonym of Apanteles plutellae Kurdjumov, 1912. Entomol Gaz 54: 187–189.
Shelton AM, Cooley RJ, Kroening MK, Wilsey MT, Eigenbrode SD (1991). Comparative analysis of two rearing procedures for diamondback moth. J Entomol Sci 26: 17–26.
Smith SG, Wallace DR (1971). Allelic sex determination in a lower hymenopteran, Neodiprion nigroscutum Midd. Can J Gen Cytol 13: 617–621.
Speicher BR, Speicher KG (1940). The occurrence of diploid males in Habrobracon brevicornis. Am Nat 74: 382–397.
Stouthamer R, Luck RF, Werren JH (1992). Genetics of sex determination and the improvement of biological control using parasitoids. Environ Entomol 21: 427–435.
Takahashi NC, Peruquetti RC, Del Lama MA, Campos LAD (2001). A reanalysis of diploid male frequencies in euglossine bees (Hymenoptera: Apidae). Evolution 55: 1897–1899.
Tavares MG, Irsigler AST, Campos LAD (2003). Testis length distinguishes haploid from diploid drones in Melipona quadrifasciata (Hymenoptera: Meliponinae). Apidologie 34: 449–455.
van Wilgenburg E, Driessen G, Beukeboom LW (2006). Single locus complementary sex determination in Hymenoptera: an ‘unintelligent’ design? Front Zool 3: 1–15.
Whiting PW (1943). Multiple alleles in complementary sex determination of Habrobracon. Genetics 28: 365–382.
Whiting PW (1960). Polyploidy in Mormoniella. Genetics 45: 949–970.
Woyke J (1963). What happens to diploid drone larvae in a honeybee colony? J Apic Res 2: 73–75.
Woyke J (1973). Artificial insemination of Apis cerana indica queens. J Apic Res 12: 151–158.
Woyke J (1974). Genic balance, heterozygosity and inheritance of testis size in diploid drone honeybees. J Apic Res 13: 77–85.
Wu Z, Hopper KH, Ode PJ, Fuester RW, Chen JH, Heimpel GE (2003). Complementary sex determination in hymenopteran parasitoids and its implications for biological control. Entomol Sin 10: 81–93.
Yamauchi K, Yoshida T, Ogawa T, Itoh S, Ogawa Y, Jimbo S et al. (2001). Spermatogenesis of diploid males in the formicine ant, Lasius sakagamii. Insect Soc 48: 28–32.
Zhou Y, Gu H, Dorn S (2006). Single-locus sex determination in the parasitoid wasp Cotesia glomerata (Hymenoptera: Braconidae). Heredity 96: 487–492.
Zhou Y, Gu H, Dorn S (2007). Effects of inbreeding on fitness components of Cotesia glomerata, a parasitoid wasp with single-locus complementary sex determination (sl-CSD). Biol Control 40: 273–279.
Acknowledgements
We thank Dominique Bordat for supplying C. vestalis from Benin, Lynn Knutson and Beth Sandager for rearing assistance, Greg Veltri of the Flow Cytometry Core Lab at the University of Minnesota Cancer Center for his help in setting up the flow cytometry analyses and Zhishan Wu for letting us use facilities at the University of Minnesota/Minnesota Department of Agriculture Quarantine Facility. This study was supported by the US National Science Foundation and the University of Minnesota Agricultural Experiment Station.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
de Boer, J., Ode, P., Vet, L. et al. Diploid males sire triploid daughters and sons in the parasitoid wasp Cotesia vestalis. Heredity 99, 288–294 (2007). https://doi.org/10.1038/sj.hdy.6800995
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.hdy.6800995
Keywords
This article is cited by
-
Co-occurrence of thelytokous and bisexual Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) in a natural population
Scientific Reports (2019)
-
Sterile males in a parasitoid wasp with complementary sex determination: from fitness costs to population extinction
BMC Ecology (2015)
-
Population-level consequences of complementary sex determination in a solitary parasitoid
BMC Evolutionary Biology (2015)
-
Selection for early emergence, longevity and large body size in wingless, sib-mating ant males
Behavioral Ecology and Sociobiology (2013)
-
Gene copy number and differential gene expression in haploid and diploid males of the stingless bee, Melipona quadrifasciata
Insectes Sociaux (2012)