Abstract
Purpose
To assess the cost-effectiveness of latanoprost or timolol in glaucoma treatment in Norway, Sweden, Denmark (Scandinavia) and the United Kingdom (UK).
Methods
A Markov model was constructed to perform a cost-effectiveness analysis. Health states were ‘stable’ and ‘progressed’ glaucoma, and transition probabilities for both primary open-angle and exfoliation glaucoma were derived from the medical literature. Practice patterns were obtained from surveys completed by 54 ophthalmologists geographically dispersed throughout each country. Country specific unit costs were used for medications, patient visits, diagnostics, and therapeutic procedures.
Results
Over the life of the model latanoprost was less expensive than timolol by 5.3–7.6% (Scandinavia) and 2.1% (UK). Following adjustments, therapy in the original timolol-treated cohort was slightly more effective in each country with a difference in 0.003–0.015 years to progression of glaucoma existing between latanoprost. This may have resulted from the model design, which reflected that physicians ultimately control most patients' glaucoma over 5 years by adding or changing therapy. The associated incremental cost-effectiveness ratios for latanoprost vstimolol generated by the Scandinavian and the UK models, respectively, were: Norway 351 396 NOK; Sweden 988 985 SEK; Denmark 351 641; and the UK 4751 GBP.
Conclusions
Over 5 years, in the UK timolol is the cost-effective option, whereas in Scandinavia latanoprost may be the cost-effective alternative to timolol.
Similar content being viewed by others
Introduction
The most commonly used first-line agents worldwide to treat glaucoma are timolol maleate and the F2α prostaglandin analogues. Generally, prostaglandin analogues are believed to have the advantage over timolol of being more efficacious and more convenient to dose.1
However, in Europe timolol is frequently prescribed as first-line therapy since, due to the availability of generics, it generally is less expensive per bottle than the prostaglandin analogs. However, other factors exist that might increase the cost of timolol therapy long-term. Previous studies have shown that latanoprost (Xalatan™, Pfizer Inc., New York, NY, USA), the first prostaglandin analogue made available, is a more persistent therapy (remaining unaltered) compared to timolol.1, 2, 3, 4, 5, 6 Consequently, treatment with timolol more often may necessitate therapy changes potentially due to side effects or compliance problems.1 In addition, since the prostaglandin analogs are more effective in reducing the intraocular pressure, timolol treatment might require the addition of a second medication.7
Accordingly, both the lower persistency rate and efficacy might make timolol a more expensive long-term therapeutic option than the prostaglandin analogs. These additional long-term costs might result from extra patient visits, diagnostics, as well as glaucoma medicines and therapeutic procedures.1 Unfortunately, little previous data have evaluated the prostaglandin analogs compared to timolol with regard to long-term clinical outcomes and cost of therapy.
The purpose of this study was to assess the long-term cost-effectiveness of latanoprost and timolol as monotherapy in the treatment of open-angle glaucoma in Norway, Sweden, Denmark (Scandinavia) and the United Kingdom (UK) by use of a Markov decision-analytic health model.
Materials and methods
Procedures
A Markov model was created to assess the cost-effectiveness in association with the quality of life gained (quality-adjusted life years [QALY]).8 This type of model has the advantage in analysing medical situations where the treatment outcomes and costs may be dissimilar. Consequently, the model allows determination of cost per unit of benefit of treatment. The unit of benefit chosen for glaucoma was the prevention of disease progression as defined by further thinning of the optic nerve head or worsening of the visual field (ie, nasal step, or arcuate, paracentral or Seidel's scotoma).9, 10, 11 The time horizon for our model was 5 years.
Markov model: medical aspects
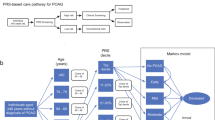
The Markov model was developed using TreeAge Pro Healthcare software (release 1.5, TreeAge Software Inc., Williamstown, MA, USA). The model analysed the cost effectiveness of beginning monotherapy treatment with either latanoprost or timolol. The potential of developing progressive glaucomatous long-term visual field loss over 5 years, based on previous studies evaluating progression at different mean intraocular pressure levels in primary open-angle and exfoliation glaucoma, is demonstrated in the model (see Supplementary Information).12, 13, 14, 15 Figure 1 demonstrates the model steps. Glaucomatous progression was assumed to be slow and staged over time. Patients beginning the model in year 1 were assumed to have early glaucomatous damage consistent with being begun on monotherapy.
We based our utility weights on different levels of glaucoma published by Kobelt16 using 0.84 for mild visual loss, 0.72 for advanced (the second progressed state). Since our model required an intermediate state (the first progressed state), which is not described in the literature, we used a utility derived between the two described states from Kobelt by the following formula: 84+72/2=78. Also, we used the 0.50 utility weight for legal blindness as published by Tengs and Wallace.17
We performed systematic literature reviews on Pubmed (www.ncbi.nlm.nih.gov/sites/entrez) to assure our model followed current medical practice. Specific information for each country was used when possible. If country-specific information was not available then generalized medical literature was used. Differences in progression rates between primary open-angle and exfoliation glaucoma were calculated by the known incidence of glaucoma in each country.18, 19, 20, 21, 22, 23, 24, 25, 26 To determine frequency of visits and procedures, as well as indications for laser and conventional therapy, we completed surveys of practicing ophthalmologists dispersed throughout the country; 12 in Norway, 14 in Sweden, 19 in Denmark and 9 in the UK.
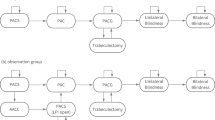
To create the model (Figure 2) we assumed that a glaucoma patient would begin either latanoprost or timolol monotherapy. The model supposed that from the initial treatment patients would enter into one of four health states: controlled (ie pressure ⩽18 mmHg) without a side effect, uncontrolled (ie pressure⩾18 mmHg) without a side effect, discontinue the medication due to a side effect and be controlled on the new medicine, or discontinue the medication due to a side effect and be uncontrolled on the new medicine.4, 7, 27, 28, 29, 30, 31 The cycle stage was assumed to be 1 year. Previously published persistency rates were used to determine discontinuation rates during the first year of therapy.1, 2, 3, 32, 33, 34, 35 We assumed that patients who discontinued timolol would be switched to latanoprost, and vice versa, and additional visits and procedures associated with the change were calculated.
The cohort of patients initially controlled (ie, pressure ⩽18 mmHg) without a side effect were assumed to be stable or progressed based on known rates in the literature.12, 13, 14, 15 We presupposed that those who were stable (no additional glaucomatous optic disc or visual field progression) maintained therapy, and remained stable, for the remaining life of the model. Those who were unstable, ie, suffered progression, received a second medication. Control rates for the new lower pressure (assumed 15 mmHg) using two medicines were presumed to be equal among all Markov branches since prior literature does not differentiate glaucoma medicines at such low levels of pressure. This adjunctive therapy cohort progressed or remained stable according to rates published previously for 15 mmHg.12, 13, 14, 15 Stable patients again were assumed to maintain their therapy, and to remain stable, for the remaining life of the model. Unstable patients, at such a low pressure, were progressed to trabeculectomy at rates determined from previously published success/failure rates.36, 37, 38, 39, 40, 41 We believed that laser trabeculoplasty would have only a minimal further hypotensive effect in a patient with a pressure of 15 mmHg, so this therapy was excluded.
The cohort of patients initially uncontrolled had a second medication added and transition probabilities for control were based on known response rates.28, 42 Those who were controlled with adjunctive therapy were assumed to maintain their therapy, and to be stable, for the remaining life of the model. Those who were uncontrolled on two medicines entered either the stable or unstable Markov branch and were assumed to undergo argon laser trabeculoplasty. The controlled cohort of these patients was considered not to progress for the remaining life of the model. Those uncontrolled after argon laser trabeculoplasty, based on rates from previous literature, were progressed to trabeculectomy at previously published success/failure rates.43, 44, 45
In addition, we assumed that a certain percentage of all patients would suffer legal blindness despite therapy. The specific rates of legal blindness were taken from the literature for Denmark and the United Kingdom and general rates were used from the United States and Europe for Norway, and Sweden. We assumed that the rates were associated for timolol since the literature was published before the release of the prostaglandin analogues.46, 47, 48, 49, 50, 51, 52, 53 We also assumed that all legal blindness occurred only in patients in the unstable branches of the Markov model. Adjustments to blindness rates were then made for each country based on the percentage of unstable patients in the latanoprost group compared to timolol.
Markov mode: economic aspects
The Markov model was completed with economic information from physician surveys and specific pricing information they use in their own practice from each country as shown in Table 1. In addition, we performed personal interviews with a practicing ophthalmologist within each Scandinavian country and the UK to obtain unit cost information for visits, procedures, and therapy. Only direct medical costs were considered (ie, cost of visits, procedures, and therapy). For timolol the cost of the generic products was used.
Costs were measured in local monetary units, and by converting currency values from Scandinavia and the UK to United States dollars at the exchange rate for 31 December 2005 (www.oanda.com; 1 Swedish Krona=0.12565 US $ 1 Norwegian Kroner=0.14750 US $ 1 Danish Krone=0.15846 US $ 1 British Pound=1.72079 US $). All costs were calculated from a payer perspective and cost results were discounted per annum at 3% for Scandinavia and 3.5% for the UK.
Markov model sensitivity analysis
For the sensitivity testing the following analyses were performed for costs: a one-way analysis of a single unit cost of latanoprost, a one-way analysis of the cost of a routine visit and a one-way analysis of the cost of blindness. In each case the cost was changed by ±10%. The analyses were performed separately for each country.
Results
Key assumptions of the study and how the Markov model was populated based on the previous literature are listed in Table 2. The cost-effectiveness results of this study are shown in Table 3. Over the life of the model latanoprost was consistently less expensive compared to timolol in each country evaluated by 5.3–7.6% in Scandinavia and 2.1% in the UK. In all four countries the average cost over the life of the model was $5339 for latanoprost and $5653 for timolol.
Differences in the long-term costs of latanoprost and timolol are at least partially explained by comparing several arms within the Markov model. First, patients originally begun on timolol and who were switched to latanoprost due to lack of persistency (higher incidence in the timolol maleate group) had higher costs (Norway 40 487 NOK; Sweden 48 430 SEK; Denmark 32 772 DKK; and the UK 3495 GBP) than patients begun and maintained on latanoprost therapy alone (Norway 37 477 NOK; Sweden 40 961 SEK; Denmark 27 814 DKK; and the UK 3032 GBP). Second, patients who were uncontrolled on timolol (higher incidence in the timolol group) had higher costs (Norway 55 605 NOK; Sweden 53 326 SEK; Denmark 41 819 DKK; and the UK 4732 GBP) than patients who were controlled on latanoprost therapy (Norway 23 884 NOK; Sweden 28 282 SEK; Denmark 20 323 DKK; and the UK 2080 GBP).
A narrow range of effectiveness 0.003–0.015 QALY existed between latanoprost and timolol. The associated incremental cost-effectiveness ratios (ICER) for latanoprost vs timolol generated by models for each country are shown in Table 3. The slightly greater QALY with timolol may have occurred because >50% of patients were not persistent on timolol and changed to latanoprost in the first year of therapy. Accordingly, for patients who maintained on their original therapy the effectiveness was greater for latanoprost (Norway 3.973; Sweden 3.955; Denmark 4.039; and the UK 4.055) compared to timolol (Norway 3.959; Sweden 3.942; Denmark 4.003; and the UK 4.009).
With none of the sensitivity tests did the ±10% change in either the cost of the latanoprost, an uncomplicated visit or blindness, influence the overall cost of therapy. In each country, for each sensitivity analysis, latanoprost remained less expensive than the timolol (ranges: Norway 297 901–404 890 NOK; Sweden 850 301–1 127 669 SEK; Denmark 349 210–413 990 DKK; and the UK 631–5201 GBP). In each country the cost of the unit bottle of latanoprost provided the greatest variance in the sensitivity testing.
Discussion
A number of economic analyses have been performed with glaucoma medicines over the past few years. Initial evaluations provided a short-term assessment of cost of therapy by simply comparing the unit bottle cost of various newer glaucoma agents. Generally these studies showed that latanoprost, bimatoprost, and travoprost had similar costs per bottle, at least within the United States.55, 56 In contrast, Costagliota et al57 found the generic timolol was approximately six times cheaper than latanoprost in Europe. However, in the United States, Stewart et al58 found that switching to latanoprost from timolol when medically indicated, compared to adding latanoprost to reduced cost over 1 year of therapy.
More recently, several papers have created economic models based on the per bottle price of the medicine and efficacy shown in the literature. These studies speculated that bimatoprost would show greater long-term cost efficiency in the United States and Europe compared to latanoprost and travoprost.59, 60, 61
Although economic modelling may be a useful tool to evaluate the long-term cost efficiency of a medicine additional factors should be considered including patient adherence to the medication, adverse events, influence of additional medical as well as surgical therapies, and the impact of long-term rates of blindness. Such factors can be evaluated in a Markov model approach to outcomes research.
Several previous studies of glaucoma medications have been evaluated by the Markov model with a time horizon of 5 years.9, 10, 11 Le Pen et al9 evaluated travoprost vs latanoprost and timolol in Austria, France, Germany, the Netherlands, and the United Kingdom. The health states were stable and progressed glaucoma. They assumed a greater efficacy with travoprost over latanoprost and timolol and they concluded that travoprost economically dominated the other medicines in several countries. Nordmannet al10 evaluated first to fourth-line treatment in glaucoma with a variety of medicines. They concluded that a more powerful first- or second-line treatment would contribute to preservation of long-term vision.
Christensen et al11 evaluated bimatoprost as an alternative to filtration surgery for glaucoma patients on maximum tolerable medical therapy in Italy over 4 years. They concluded that bimatoprost was less expensive than filtration surgery and had important implications for waiting list planning.
The purpose of this current study was to assess the long-term cost-effectiveness of latanoprost or timolol as monotherapy in the treatment of open-angle glaucoma in Scandinavia and the United Kingdom using a Markov model.
This study showed that when latanoprost was begun as monotherapy it was less expensive than timolol over 5 years in Scandinavia and the UK. This finding goes against conventional wisdom that timolol is a less expensive therapy than latanoprost based upon per unit cost (cost per month).
The reason why latanoprost was less expensive than timolol in our model can mostly be explained by several reasons: first, latanoprost, when given as monotherapy, more often controls the intraocular pressure. Accordingly, the controlled arm for latanoprost, in the Markov model, demonstrated lower long-term costs than patients in the uncontrolled arm for timolol with its accompanying higher costs for a greater number of diagnostics, visits medicines, and surgeries. Second, patients on latanoprost were more likely to be persistent than those on timolol. Accordingly, in the Markov model latanoprost-treated patients who began and maintained this medicine demonstrated lower costs than those initiated on timolol and were switched later to the prostaglandin therapy with the associated extra costs for visits and procedures. Finally, because patients were more often stable on latanoprost they were less likely to go blind in the model with its associated additional costs.
The narrow increase of effectiveness with the original cohort of timolol-treated patients compared to latanoprost over the 5 years was a surprise to the authors. However, the gain in quality adjusted life year in each country with timolol represented by these findings was small (0.003–0.015 QALYs). The extra cost per QALY gained in Scandinavia and the UK was from $8175 to $124 270. Although controversial, the National Institute for Clinical Excellence (NICE) stated that if an ICER is lower than 33 000–50 000 euros ($39 087–$59 223) the strategy could be considered effective and a specific resource allocation could be made available.62 The QALYs in our study did not appear close to the NICE definition in Scandinavia. However, in the UK the ICER was $8175, which falls under the NICE definition. On a practical basis, however, the small gain in quality-adjusted life year (5 days) in the UK, for the additional cost, does not seem worthwhile. Nonetheless, no set standards are available generally to guide physicians to the value of glaucoma treatments based on ICER levels.
Why timolol was more cost-effective in the UK long-term compared to Scandinavia is not clear by our results. Several potential explanations were that timolol generic was even less expensive in the UK, than in Scandinavia. This factor may have further benefited the cost-utility of this timolol in the UK. In addition, the UK had a markedly lower incidence of exfoliation glaucoma compared to Scandinavia. Because of the higher rate of blindness and the more elevated intraocular pressures with exfoliation glaucoma it could be that the use of a prostaglandin first line provides a greater cost-benefit ratio long-term in Scandinavia specifically to better control this more difficult glaucoma.
The slightly greater effectiveness of timolol over latanoprost in this study most likely resulted from the design which recognized that a physician, over the long-term course of a patient's disease, will adjust their therapy to reach a chosen target pressure and to limit side effects.12, 13, 14 Accordingly, based on the literature, within the first year of therapy a majority of patients started on timolol were switched to latanoprost.1, 2, 3, 7 Consequently, these patients were able to gain the efficacy and advantage of prostaglandin therapy.
The evaluation of the effectiveness for the latanoprost and timolol arms, which excluded patients who were switched to the other medicine because of lack of persistency, is instructive. These arms represent the pure effectiveness of latanoprost or timolol since they are not switched to the other medication. The effectiveness for the latanoprost arm in this portion of the model was greater than timolol. This finding is consistent with the majority of past studies which demonstrated that latanoprost is a more effective ocular hypotensive agent than timolol.7, 63, 64, 65, 66 For the above reasons, this study does not support the greater effectiveness of timolol over latanoprost.
Economic sensitivity testing for the costs of the latanoprost bottle, blindness, and visits added little to the results in each country. Even a 10% greater unit cost for latanoprost did not alter the basic finding that, for each country, latanoprost was a less expensive long-term therapeutic option compared to timolol.
This study suggests that over the course of 5 years, in the United Kingdom timolol is the most cost-effective option, whereas in Scandinavia latanoprost may be the cost-effective alternative to timolol.
Importantly, the findings of this study resulted from a model that is based on clinical data from the literature and do not represent results from actual patients. This study did not evaluate the long-term clinical effectiveness and cost of latanoprost, and timolol in a prospective, randomized, double-masked manner. In addition, the model, to limit over complexity had to make assumptions, such as a stable year period between health states. Such assumptions limit the accuracy of the Markov model to real clinical settings. Further, more specific utility weights related to the various degenerative stages of glaucoma are needed to help create a more accurate Markov model for this disease state. Care should be utilized by the physician in applying modelled information, based on limited assumptions to clinical practice. Modelled data should not supersede clinical judgment. More research is needed in a prospective, long-term design, using a naturalistic follow-up approach, to more accurately describe the long-term differences in cost and effectiveness between latanoprost and timolol.
References
Day DG, Schacknow PN, Sharpe ED, Ellyn JC, Kulze JC, Threlkeld AB et al. A persistency and economic analysis of latanoprost, bimatoprost or beta-blockers in patients with open-angle glaucoma or ocular hypertension. J Ocul Pharmacol Ther 2004; 20: 383–392.
Reardon G, Schwartz GF, Mozaffari E . Patient persistency with topical ocular hypotensive therapy in a managed care population. Am J Ophthalmol 2004; 137: S3–S12.
Schwartz GF, Reardon G, Mozaffari E . Persistency with latanoprost or timolol in primary open-angle glaucoma suspects. Am J Ophthalmol 2004; 137: S13–S16.
Sherwood M, Brandt J, Bimatoprost Study Groups 1 and 2. Six-month comparison of bimatoprost once-daily and twice-daily with timolol twice-daily in patients with elevated intraocular pressure. Surv Ophthalmol 2001; 45: S361–S368.
Netland PA, Landry T, Sullivan K, Andrew R, Silver L, Weiner A et al. Travoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertension. Am J Ophthalmol 2001; 132: 472–484.
Zhang WY, Po AL, Dua HS, Azuara-Blanco A . Meta-analysis of randomised controlled trials comparing latanoprost with timolol in the treatment of patients with open angle glaucoma or ocular hypertension. Br J Ophthalmol 2001; 85: 983–990.
Hedman K, Larsson LI . The effect of latanoprost compared with timolol in African-American, Asian, Caucasian, and Mexican open-angle glaucoma or ocular hypertensive patients. Surv Ophthalmol 2002; 47: S77–S89.
Rychlik R . Strategies in Pharmacoeconomics and Outcomes Research. Hayworth Press Inc.: Binghampton, NY, 2002.
Le Pen C, Ligier M, Berdeaux G . Cost-effectiveness and cost-utility analysis of travoprost versus latanoprost and timolol in the treatment of advanced glaucoma in five European countries: Austria, France, Germany, The Netherlands and the United Kingdom. J Med Econ 2005; 8: 67–84.
Nordmann JP, Lafuma A, Deschaseaux C, Berdeaux G . Clinical outcomes of glaucoma treatments over a patient lifetime: a Markov model. J Glaucoma 2005; 14: 463–469.
Christensen TL, Poulsen PB, Holmstrom S, Walt JG, Vetrugno M . A Markov modeled pharmacoeconomic analysis of bimatoprost 0.03% in the treatment of glaucoma as an alternative to filtration surgery in Italy. Curr Med Res Opin 2005; 21: 1837–1844.
Mao LK, Stewart WC, Shields MB . Correlation between intraocular pressure control and progressive glaucomatous damage in primary open-angle glaucoma. Am J Ophthalmol 1991; 111: 51–55.
Stewart WC, Chorak RP, Hunt HH, Sethuraman G . Factors associated with visual loss in patients with advanced glaucomatous changes in the optic nerve head. Am J Ophthalmol 1993; 116: 176–181.
Stewart WC, Kolker AE, Sharpe ED, Day DG, Holmes KT, Leech JN et al. Factors associated with long-term progression or stability in primary open-angle glaucoma. Am J Ophthalmol 2000; 130: 274–279.
Konstas AG, Hollo G, Astakhov YS, Teus MA, Akopov EL, Jenkins JN et al. Factors associated with long-term progression or stability in exfoliation glaucoma. Arch Ophthalmol 2004; 122: 29–33.
Kobelt G, Jonsson B, Bergstrom A, Chen E, Linden C, Alm A . Cost-effectiveness analysis in glaucoma: what drives utility? Results from a pilot study in Sweden. Acta Ophthalmol Scand 2006; 84: 363–371.
Tengs TO, Wallace A . One thousand health-related quality-of-life estimates. Med Care 2000; 38: 583–637.
Blika S, Ringvold A . The occurrence of simple and capsular glaucoma in Middle-Norway. Acta Ophthalmol Scan Suppl 1987; 182: S11–S16.
Ringvold A, Blika S, Elsas T, Guldahl J, Brevik T, Hesstvedt P et al. The middle-Norway eye-screening study. II. Prevalence of simple and capsular glaucoma. Acta Ophthalmol (Copenh) 1991; 69: 273–280.
Lindblom B, Thorburn W . Observed incidence of glaucoma in Halsingland, Sweden. Acta Ophthalmol (Copenh) 1984; 62: 217–222.
Grodum K, Heijl A, Bengtsson B . A comparison of glaucoma patients identified through mass screening and in routine clinical practice. Acta Ophthalmol Scan 2002; 80: 627–631.
Ekstrom C . Elevated intraocular pressure and pseudoexfoliation of the lens capsule as risk factors for chronic open-angle glaucoma. A population-based five-year follow-up study. Acta Ophthalmol (Copenh) 1993; 71: 189–195.
Ohrt V, Hehen JH . The incidence of glaucoma capsulare based on a Danish hospital material. Acta Ophthalmol Scand 1981; 59: 888–893.
Alyahya GA, Hietanen J, Heegaard S, Kivela T, Prause JU . Exfoliation syndrome in Nordic countries: a comparative histopathological study of Danish and Finnish eyes with absolute glaucoma and uveal melanoma. Acta Ophthalmol Scand 2005; 83: 711–715.
Aasved H . Prevalence of fibrillopathia epitheliocapsularis (pseudoexfoliation) and capsular glaucoma. Trans Ophthalmol Soc UK 1979; 99: 293–295.
Aasved H . The geographical distribution of fibrillopathia epitheliocapsularis, so-called senile exfoliation or pseudoexfoliation of the anterior lens capsule. Acta Ophth (Copenh) 1969; 47: 792–810.
Noecker RS, Dirks MS, Choplin NT, Bernstein P, Batoosingh AL, Whitcup SM . A six-month randomized clinical trial comparing the intraocular pressure-lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J Ophthalmol 2003; 135: 55–63.
Higginbotham EJ, Schuman JS, Goldberg I, Gross RL, VanDenburgh AM, Chen K et al. One-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthalmol 2002; 120: 1286–1293.
Cohen JS, Gross RL, Cheetham JK, VanDenburgh AM, Bernstein P, Whitcup SM . Two-year double-masked comparison of bimatoprost with timolol in patients with glaucoma or ocular hypertension. Surv Ophthalmol 2004; 49: S45–S52.
Parrish RK, Palmberg P, Sheu WP, XLT Study Group. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol 2003; 135: 688–703.
Hedman K, Alm A . A pooled-data analysis of three randomized, double-masked, six-month clinical studies comparing the intraocular pressure reducing effect of latanoprost and timolol. Eur J Ophthalmol 2000; 10: 95–104.
Zhou Z, Althin R, Sforzolini BS, Dhawan R . Persistency and treatment failure in newly diagnosed open angle glaucoma patients in the United Kingdom. Br J Ophthalmol 2004; 88: 1391–1394.
Dasgupta S, Oates V, Bookhart BK, Vaziri B, Schwartz GF, Mozaffari E . Population-based persistency rates for topical glaucoma medications measured with pharmacy claims data. Am J Manag Care 2002; 8: S255–S261.
Spooner JJ, Bullano MF, Ikeda LI, Cockerham TR, Waugh WJ, Johnson T et al. Rates of discontinuation and change of glaucoma therapy in a managed care setting. Am J Manag Care 2002; 8: S262–S270.
Reardon G, Schwartz GF, Mozaffari E . Patient persistency with ocular prostaglandin therapy: a population-based, retrospective study. Clin Ther 2003; 25: 1172–1185.
The Fluorouracil Filtering Surgery Study Group. Five-year follow-up of the Fluorouracil Filtering Surgery Study. Am J Ophthalmol 1996; 121: 349–366.
Rothman RF, Liebmann JM, Ritch R . Low-dose 5-fluorouracil trabeculectomy as initial surgery in uncomplicated glaucoma: long-term follow-up. Ophthalmology 2000; 107: 1184–1190.
Marquardt D, Lieb WE, Grehn F . Intensified postoperative care versus conventional follow-up: a retrospective long-term analysis of 177 trabeculectomies. Graefes Arch Clin Exp Ophthalmol 2004; 242: 106–113.
Wickham MG, Worthen DM . Argon laser trabeculotomy: long-term follow-up. Ophthalmology 1979; 86: 495–503.
Parrish II RK, Schiffman JC, Feuer WJ, Heuer DK, Fluorouracil Filtering Surgery Study Group. Prognosis and risk factors for early postoperative wound leaks after trabeculectomy with and without 5-fluorouracil. Am J Ophthalmol 2001; 132: 633–640.
Diestelhorst M, Khalili MA, Krieglstein GK . Trabeculectomy: a retrospective follow-up of 700 eyes. Int Ophthalmol 1998–99; 22: 211–220.
Pfeiffer N, European Latanoprost Fixed Combination Study Group. A comparison of the fixed combination of latanoprost and timolol with its individual components. Graefes Arch Clin Exp Ophthalmol 2002; 240: 893–899.
Chung PY, Schuman JS, Netland PA, Lloyd-Muhammad RA, Jacobs DS . Five-year results of a randomized, prospective, clinical trial of diode vs argon laser trabeculoplasty for open-angle glaucoma. Am J Ophthalmol 1998; 126: 185–190.
Odberg T . Primary laser trabeculoplasty in glaucoma. A resource saving treatment. Tidsskr Nor Laegeforen 1990; 110: 3729–3730.
Spaeth GL, Baez KA . Argon laser trabeculoplasty controls one third of cases of progressive, uncontrolled, open angle glaucoma for 5 years. Arch Ophthalmol 1992; 110: 491–494.
Rosenberg T, Klie F . Current trends in newly registered blindness in Denmark. Acta Ophthalmol Scand 1996; 74: 395–398.
Fuchs J, Nissen KR, Goldschmidt E . Glaucoma blindness in Denmark. Acta Ophthalmol (Copenh) 1992; 70: 73–78.
Quigley HA, Vitale S . Models of open-angle glaucoma prevalence and incidence in the United States. Invest Ophthalmol Vis Sci 1997; 38: 83–91.
Congdon N, O'Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004; 122: 477–485.
Chen PP . Blindness in patients with treated open-angle glaucoma. Ophthalmology 2003; 110: 726–733.
Hattenhauer MG, Johnson DH, Ing HH, Herman DC, Hodge DO, Yawn BP et al. The probability of blindness from open-angle glaucoma. Ophthalmology 1998; 105: 2099–2104.
Friedman DS, West SK, Munoz B, Park W, Deremeik J, Massof R et al. Racial variations in causes of vision loss in nursing homes: The Salisbury Eye Evaluation in Nursing Home Groups (SEEING) Study. Arch Ophthalmol 2004; 122: 1019–1024.
Kocur I, Resnikoff S . Visual impairment and blindness in Europe and their prevention. Br J Ophthalmol 2002; 86: 716–722.
Meads C, Hyde C . What is the cost of blindness? Br J Ophthalmol 2003; 87: 1201–1204.
Fiscella RG, Green A, Patuszynski DH, Wilensky J . Medical therapy cost considerations for glaucoma. Am J Ophthalmol 2003; 136: 18–25.
Mick AB, Gonzalez S, Dunbar MT, McSoley JJ . A cost analysis of the prostaglandin analogs. Optometry 2002; 73: 614–619.
Costagliola C, Parmeggiani F, Sebastiani A . Assessing the cost-effectiveness of switching from a beta-blocker to latanoprost in the treatment of ocular hypertension. Expert Opin Pharmacother 2003; 4: 1775–1788.
Stewart WC, Leech J, Sharpe ED, Kulze J, Ellyn J, Day DG . An economic analysis of switching to latanoprost from a beta-blocker or adding brimonidine or latanoprost to a beta-blocker in open-angle glaucoma or ocular hypertension. Am J Manag Care 2002; 8: S240–S248.
Goldberg LD, Walt J . Cost considerations in the medical management of glaucoma in the US: estimated yearly costs and cost effectiveness of bimatoprost compared with other medications. Pharmacoeconomics 2006; 24: 251–264.
Noecker RJ, Walt JG . Cost-effectiveness of monotherapy treatment of glaucoma and ocular hypertension with the lipid class of medications. Am J Ophthalmol 2006; 141: S15–S21.
Tuil E, Hommer AB, Poulsen PB, Christensen TL, Buchholz P, Walt J et al. The cost-effectiveness of bimatoprost 0.03% in the treatment of glaucoma in adult patients – a European perspective. Int J Clin Pract 2005; 59: 1011–1016.
National Institute for Clinical Excellence. Guide to the Methods of Technology Appraisal. NICE: London, UK, 2004.
Alm A, Stjernschantz J, Scandinavian Latanoprost Study Group. Effects on intraocular pressure and side effects of 0.005% latanoprost applied once daily, evening or morning: a comparison with timolol. Ophthalmology 1995; 102: 1743–1752.
Camras CB, United States Latanoprost Study Group. Comparison of latanoprost and timolol in patients with ocular hypertension and glaucoma: a six-month, masked, multicenter trial in the United States. Ophthalmology 1996; 103: 138–147.
Watson P, Stjernschantz J, Latanoprost Study Group. A six-month, randomized, double-masked study comparing latanoprost with timolol in open-angle glaucoma and ocular hypertension. Ophthalmology 1996; 103: 126–137.
Stewart WC, Day DG, Sharpe ED, Dubiner HB, Holmes KT, Stewart JA . Efficacy and safety of timolol solution once daily vs timolol gel added to latanoprost. Am J Ophthalmol 1999; 128: 692–696.
Acknowledgements
This study was supported by an unrestricted grant from Pfizer Inc., New York, NY, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stewart, W., Stewart, J. & Mychaskiw, M. Cost-effectiveness of latanoprost and timolol maleate for the treatment of glaucoma in Scandinavia and the United Kingdom, using a decision-analytic health economic model. Eye 23, 132–140 (2009). https://doi.org/10.1038/sj.eye.6702964
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702964
Keywords
This article is cited by
-
Manufacturing and characterisation of 3D-printed sustained-release Timolol implants for glaucoma treatment
Drug Delivery and Translational Research (2024)
-
Systematic methodological review of health state values in glaucoma cost-utility analyses
The European Journal of Health Economics (2024)
-
Systematic Review of Economic Evaluations in Primary Open-Angle Glaucoma: Decision Analytic Modeling Insights
PharmacoEconomics - Open (2020)
-
Response to Anderson et al.
Eye (2009)