Abstract
Aim
To compare Stratus-OCT measurements in controls, ocular hypertensive (OHT) patients with (FDT+) and without (FDT−) frequency-doubling technology (FDT) abnormalities, and in patients affected with early primary open-angle glaucoma (POAG).
Methods
Thirty-two controls, 78 OHT patients (38 FDT− and 40 FDT+), and 45 early POAG patients (six FDT− and 39 FDT+) underwent the following tests within 3 months: standard automated perimetry (SAP) HFA 30–2; FDT N-30-F; and, Stratus-OCT imaging with retinal nerve fibre layer (RNFL) and optic nerve head (ONH) scans. One eye per patient was considered. Differences among groups were evaluated using the Kruskal–Wallis, analysis of variance, and Duncan's tests.
Results
There were no significant differences in all Stratus-OCT parameters between POAG and OHT FDT+ patients. Statistically significant differences were found between the control group and both the POAG and OHT FDT+ groups for 15 of the 21 Stratus-OCT parameters. Control eyes compared to OHT FDT− showed significant differences in 13 of the 21 parameters. The comparison between the OHT FDT− group, and both the POAG and OHT FDT+ group resulted in 13 of the 21 parameters to be significantly different.
Conclusions
Stratus-OCT seems to show a higher ability in detecting significant differences between healthy, OHT, and early POAG eyes when compared to SAP and FDT. This suggests that the Stratus-OCT could show structural abnormalities before SAP or FDT visual field defects appear in patients at risk of developing glaucoma, which may be beneficial in making therapeutic decisions, especially in OHT patients.
Similar content being viewed by others
Introduction
The diagnosis of glaucoma is currently based on the appearance of the optic nerve head (ONH) and standard automated perimetry (SAP) testing results.1 Histological studies have demonstrated that 30–50% of the retinal ganglion cells may be lost before visual field (VF) defects appear on SAP.2 Reports have also shown that subtle structural ONH and peripapillary retinal nerve fibre layer (RNFL) defects can precede the development of detectable SAP VF loss in cases of early glaucomatous optic neuropathy (GON).2, 3 Several techniques have been introduced over the past years aiming at detecting morphological and functional glaucomatous abnormalities earlier than conventional methods.
Frequency-doubling technology (FDT) is a nonconventional technique used to examine the VF developed in 1997, with the aim of detecting functional damage before SAP.4, 5, 6, 7 FDT is based on the frequency-doubling (FD) illusion phenomenon first described by Kelly,8 in which a sinusoidal grating of low spatial frequency and high temporal frequency produces a perceived image that is twice its actual spatial frequency. Some authors believe that FDT selectively analyses a subset of the magnocellular pathway, known as My cells,9 whereas others question the mere existence of these cells in humans and believe this may be due to a more complicated processing that goes beyond a retinal level.10 FDT has shown to offer good sensitivity and specificity in the detection of early glaucoma,11, 12, 13, 14 and it has been reported to be a good predictor of future SAP VFs defects.15, 16, 17, 18, 19
Optical coherence tomography (OCT), first described in 1991 by Huang et al,20 is a noncontact, high-resolution technique using a scanning interferometer to produce cross-sectional images of the retina and peripapillary RNFL in vivo.20, 21 Previous OCT versions have been shown to be useful in glaucoma diagnosis.12, 21, 22, 23, 24, 25 Stratus-OCT (Carl Zeiss Meditec Inc., Dublin, CA, USA) is the latest version released in 2003, offering better axial resolution (8–10 μm), increased reproducibility, and ONH topography analysis for glaucoma diagnosis and follow-up.26, 27, 28, 29, 30 Studies have shown enhanced discrimination with the Stratus-OCT for global RNFL thickness.31 Both FDT and OCT have shown higher ability in the early detection of glaucoma than SAP.18, 32
The aim of our study was to compare Stratus-OCT measurements between controls, ocular hypertensive (OHT) patients either with or without FDT defects, and patients affected with early primary open-angle glaucoma (POAG), in order to determine how well Stratus-OCT topographic parameters work in differentiating among these conditions.
Materials and methods
This observational cross-sectional study included 155 consecutive subjects: 78 patients with OHT, 45 with POAG, and 32 controls. Glaucoma patients were recruited from the glaucoma clinic at the Ophthalmological Department of the S Maria della Misericordia Hospital, Udine, Italy. Control subjects were recruited from staff members and volunteers. The research was conducted following the guidelines of the Tenets of the Declaration of Helsinki. Institutional Review Board (IRB) approval was obtained for the study. After obtaining informed consent, all subjects underwent the following: complete ophthalmologic examination; central corneal thickness (CCT) measurements; SAP and FDT testing in random order; and Stratus-OCT imaging of the ONH and RNFL. All examinations were conducted within a 3-month period. To avoid confounding factors between eyes, only one eye per patient was randomly selected for analysis when both eyes satisfied the entry criteria.
The inclusion criteria included: best-corrected visual acuity (BCVA) ≥0.7; open anterior chamber angle; absence of ocular pathologies other than glaucoma; reliable SAP and FDT test results; and good OCT image quality. The exclusion criteria included: refractive error >±5 diopters; pupils <3 mm in diameter anterior angle alterations; presence of secondary causes of glaucoma; advanced glaucomatous VF defects; papillary anomalies; large peripapillary atrophy; media opacities precluding OCT scanning; previous intraocular surgery, diabetes mellitus, neurological disorders, and medication that could modify perimetric results.
CCT was measured with ultrasonic pachymetry (Altair pachymeter, Optikon 2000; Rome, Italy). In order to avoid false-positive OHT patients, all GAT measurements were corrected on the basis of the CCT value according to the formula proposed by Doughty and Zaman:33 corrected GAT=measured GAT−[(CCT-535) × (2.5/50)].
SAP testing was performed using the Humphrey field analyzer (HFA) II 750 (Carl Zeiss Meditec Inc., Dublin, CA, USA) 30-2 test with standard Swedish interactive threshold algorithm (SITA) strategy. A normal SAP testing result was defined as a mean deviation (MD) and pattern standard deviation (PSD) within 95% confidence limits, and a glaucoma hemifield test (GHT) result ‘within normal limits’. SAP tests were classified as glaucomatous according to the Anderson and Patella34 criteria, in which at least one of the following was present in two consecutive examinations: (1) a cluster of ⩾3 points in the pattern deviation probability (PDP) plot, located in areas that are typical of glaucoma, having a probability level of P≤5%, with at least one point having a probability level of P≤1%; none of the points could be edge-points unless they were located immediately above or below the nasal horizontal meridian; (2) PSD with a probability level of P<5%; (3) GHT ‘outside normal limits’. Reliable criteria for HFA tests included false-positive and false-negative responses <33% and fixation losses <20%. Only early glaucomatous SAP VF defects, having a MD better than −5.0 dB and a PSD<5.0 dB, were included.
FDT was performed with the FDT Visual Field Instrument (Welch Allyn FDT, Skaneateles Falls, NY, USA and Carl Zeiss Meditec Inc., Dublin, CA, USA) N-30 full-threshold test that has been described elsewhere.4, 7 In brief, the FDT test stimulus consists of a sinusoidal grating of low spatial frequency, undergoing counter phase flicker at high temporal frequency at different contrast levels. The threshold value for each test location is defined as the minimal contrast at which the stimulus is perceived. The FDT N-30 test includes 19 stimuli (18 square 10° × 10° targets and one central 5° × 5° circular target) having a spatial frequency of 0.25 cycles/degree and a temporal frequency of 25 Hz. Only reliable FDT results were considered, which included fixation loss, false-negative and false-positive responses, all less than 2. According to the criteria proposed by Medeiros et al,35 FDT results were considered abnormal if PSD<5% and/or ⩾2 areas with P<5% on the PDP plot were present. These criteria resulted in a specificity of 93.7% in the normal control group.
The patients were classified into the following four groups:
-
1)
control group (32 eyes): IOP⩽21 mm Hg, normal ONH and RNFL appearance (no diffuse or focal rim thinning, cupping, optic disc haemorrhage, or RNFL defects), SAP and FDT within normal limits, and no family history of glaucoma or any ocular pathologies;
-
2)
OHT without FDT abnormalities (OHT FDT− group) (38 eyes): IOP>21 mm Hg, normal SAP and FDT results;
-
3)
OHT with FDT abnormalities (OHT FDT+ group) (40 eyes): IOP>21 mm Hg, normal SAP and abnormal FDT results in at least two consecutive tests;
-
4)
POAG group (45 eyes): IOP>21 mm Hg before medication, and reproducible glaucomatous SAP VF defects in at least two consecutive tests.
In order to avoid bias in the evaluation of the Stratus-OCT diagnostic ability, patients were classified as having OHT or glaucoma on the basis of the IOP measurement and SAP results, regardless of the appearance of the ONH and RNFL.36
OCT imaging was performed using Stratus-OCT (Carl Zeiss Meditec Inc., Dublin, CA, USA; software version 3.0). OCT principles have already been reported.20 OCT scans of the ONH and RNFL were obtained using the ‘Fast optic disc scan’ and the ‘Fast RNFL thickness 3.46 scan’ protocols, respectively. ONH scans were generated from six 6-mm radial linear scans, 30° apart, each composed of 128 A-scan points, with mathematical interpolation to fill the gaps between the rays. The ONH margin automatically defined by the OCT software was used in order to avoid any type of subjective component.25 The RNFL scan was generated from the mean of three 360° circular 3.46 mm-diameter circumpapillary scans centred on the ONH. Each scan was derived from 256 A-scan points distributed along the circular circumference. Poor-quality OCT scans were defined as those having a signal-to-noise ratio <40 dB or the presence of overt misalignment of the surface detection algorithm of at least 15 consecutive pixels or 20 cumulative pixels. Stratus-OCT parameters considered in our analysis included: eight parameters listed on the ‘ONH analysis results’; 11 parameters listed on the ‘RNFL thickness average analysis report’; and the mean thickness values of the nasal (136–225°) and temporal (316–45°) quadrants. Left eye results were converted in a right eye format for the analysis. Comparisons among the groups were made using the Kruskal–Wallis test and analysis of variance, and corrected for age using the general linear model procedure (in which age was processed as a covariate factor). Pair-wise multiple comparisons were made using Duncan's multiple range test. A P-value <0.05 was considered to be statistically significant. SPSS 11.0 program package (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis.
Results
The population demographics, CCT, SAP, and FDT results are listed in Table 1. Stratus-OCT parameters for both the Fast optic disc scan and for the Fast RNFL thickness scan that were all considered in the analysis are listed in Table 2. The P-values of the differences between groups (Duncan's multiple range test) for all Stratus-OCT parameters are listed in Table 3.
Control subjects were significantly younger than the other groups (Kruskal–Wallis test, P<0.02). SAP MD and PSD were highest in the POAG group; FDT MD and FDT PSD were greater in both the POAG and OHT FDT+ groups (Duncan's test, P<0.01). The mean CCT was significantly lower in the OHT FDT+ group (Duncan's test, P<0.05).
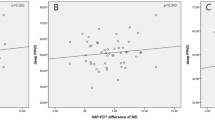
With regards to Stratus-OCT parameters (Tables 2 and 3, Figures 1 and 2), there were no significant differences in all OCT parameters between POAG and OHT FDT+ eyes. Thirteen out of 21 parameters were significantly different when the OHT FDT− group was compared to both the POAG and OHT FDT+ groups (Table 3, Duncan's test, P<0.05). Statistically significant differences (Table 3, Duncan's test, P<0.05) were also found for most parameters (15 of 21) when comparing the controls to the POAG and OHT FDT+ groups, with exception to disc area and all thickness ratio parameters (Imax/Smax, Smax/Imax, Smax/Tavg, Imax/Tavg, Smax/Navg). Statistically significant differences were found between control and OHT FDT− eyes for 13 of 21 parameters (Table 3, Duncan's test, P<0.05).
Discussion
The present study aims to evaluate the presence of FDT and OCT abnormalities in controls, OHT and early POAG patients. Although one method analyses structure, the other pertains to function, comparisons between these two testing devices can prove to be beneficial, especially considering that both diagnostic techniques have been shown to be able to detect glaucomatous damage earlier than SAP.14, 18, 32 FDT results in our study were used as the criterion for discriminating those OHT eyes at risk of developing glaucoma, considering that several longitudinal studies have demonstrated that FDT can detect signs of glaucomatous damage several years before the onset of typical SAP defects.17, 18 Furthermore, a recent prospective longitudinal study by Wollstein et al,37 which used an OCT prototype device, reported a greater likelihood of glaucomatous progression identified by OCT vs SAP, and concluded that OCT may be predictive of the future onset of SAP VF loss in patients at risk of glaucoma.
To the best of our knowledge, this is the first study designed to compare Stratus-OCT measurements in OHT patients, specifically considering those with and without FDT abnormalities.
In our sample, FDT showed a sensitivity of 86.6% and a specificity of 93.7% in detecting early glaucomatous damage when using the criteria proposed by Medeiros et al.35 This is in agreement with other studies that have reported FDT sensitivity ranging from 60 to 85% with specificity set at ≥90% in patients with early glaucoma defined with SAP.7, 11, 12, 13 Six of the 45 POAG patients showed SAP abnormalities without FDT abnormalities. This may be due to several possibilities: limitations of FDT in identifying all abnormalities; selection of criteria used in our study to define abnormality; SAP and FDT analyse different VF mechanisms; and, ganglion cell damage may not necessarily affect all early glaucomatous eyes in the same way.
Statistically significant differences were found between the POAG and the OHT FDT+ groups for SAP MD and PSD, but not for FDT MD and PSD (Table 1). These data may suggest that FDT can be useful in detecting glaucomatous VF damage in patients at risk earlier than SAP and are in agreement with several studies.15, 16, 17, 18, 19 Our results showed that there were no significant differences in Stratus-OCT parameters between the POAG and OHT FDT+ groups,(Tables 2 and 3, Figure 1 and 2) and thus seem to show that eyes with early SAP abnormalities have similar structural changes to those having abnormalities strictly detected by FDT. These data suggest that Stratus-OCT has higher accordance to FDT as opposed to SAP, considering that all OHT patients had a normal SAP.
Most of the Stratus-OCT parameters (15 of 21) were significantly different between the control and POAG group (with exception to disc area and for all thickness ratio parameters). These results are in agreement with several studies that showed that both previous OCT versions,12, 21, 22, 23, 24, 25 and the current Stratus-OCT26, 27, 28 perform well in discriminating glaucomatous eyes with early SAP defects from control eyes. On the other hand, studies have shown that OCT and SAP do not correlate so well in glaucoma suspects defined as eyes with suspicious optic disc cupping and normal SAP.38, 39
Numerous studies have shown the value of earlier OCT versions and Stratus-OCT in OHT and early glaucoma.22, 32, 40, 41, 42
Our study shows that 13 out of 21 Stratus-OCT parameters were statistically significant different between OHT eyes with normal FDT results and those with FDT abnormalities (Tables 2 and 3; Figures 1 and 2). This finding suggests that patients with FDT VF defects may have more structural damage compared to those in whom no FDT defects have developed. Similar to our findings, Mok et al32 used OCT 2000 to show that glaucoma suspects with an abnormal SAP had a thinner RNFL thickness than those with a normal SAP.
Thirteen of the 21 Stratus-OCT parameters were able to differentiate between controls and OHT FDT− eyes (Tables 2 and 3; Figures 1 and 2); however, MD, PSD (Table 1), and number of locations with P<5% in the PDP plot (data not shown) of the SAP and FDT results did not show any statistically significant differences. Our findings suggest that Stratus-OCT may detect mild glaucomatous damage earlier than FDT and SAP. This is in agreement with a recent study by Bowd et al12 that reported a higher diagnostic ability in discriminating between control and glaucomatous eyes for OCT 2000 compared to FDT, regardless of whether glaucoma was defined based on SAP results or optic disc appearance. Our results are in disagreement to the study by Mistlberger et al,43 showing that there were no significant differences in OCT 2000 parameters between OHT eyes with a normal SAP from controls. Considering that SAP and FDT have shown similar predictive value in the future onset and location of SAP VF loss,17 our results seem to indicate that Stratus-OCT has higher diagnostic ability than the previous versions, and that the inclusion criteria for OHT patients must be carefully considered. Furthermore, we attempted to limit the number of false-positive OHT subjects and consider the greater risks of developing FDT glaucomatous defects in thinner corneas by using the GAT correction factor based on CCT44, 45 (see Materials and methods).
There are some limitations in our study that should be noted. Considering that aging affects the RNFL thickness and ONH topography,21 and that controls were significantly younger than the other groups (Table 1, Kruskal–Wallis test, P<0.02), the comparisons among the groups were corrected for age using the general linear model of the analysis of variance, in which age was processed as a covariate factor. There was also a form of bias in the selection of controls, considering that inclusion criteria for this group required a normal ONH appearance in biomicroscopy. This was done in order to avoid the inclusion of subjects with GON and normal VF, but may have inadvertently caused an overestimation in the diagnostic accuracy of the Stratus-OCT parameters (especially ONH parameters). One last limitation is that there is a possibility that some of the subjects with an abnormal FDT or OCT results were false-positive. It is not possible to conclude from our results that FDT and OCT are able to strictly detect early inevitable glaucomatous defects, as our result might reflect an over sensitivity of these methods in selected cases that show abnormalities, but that will probably never develop in full fledge defects. Longitudinal studies are needed to determine the true predictive values of FDT and OCT.
In conclusion, Stratus-OCT measurements seem to show significant differences when subjects without VF defects (controls and OHT FDT−) are compared to those with functional loss detected with SAP and/or FDT, suggesting that OCT offers early detection of glaucomatous structural damage. Moreover, controls and OHT without SAP or FDT VF defects showed differences in more than half of the Stratus-OCT parameters. These results indicate that Stratus-OCT could be able to detect structural defects very early before the development of functional defects in patients at risk of developing glaucoma, which may prove to be helpful in making therapeutic decisions, especially in OHT patients. Long-term prospective follow-up studies are required to confirm the ability of the OCT in predicting future SAP and FDT VF defects.
References
American Academy of Ophthalmology preferred practice patterns committee glaucoma panel. Preferred practice patterns. Primary open angle glaucoma suspect. San Francisco: American Academy of Ophthalmology, 2002.
Sommer A, Katz J, Quigley HA, Miller RN, Robin AL, Richter RC et al. Clinically detectable nerve fiber layer atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol 1991; 109: 77–83.
Johnson CA, Cioffi GA, Liebmann JR . The relationship between structural and functional alterations in glaucoma: a review. Semin Ophthalmol 2000; 4: 221–233.
Johnson C, Samuels S . Screening for glaucomatous visual field loss with frequency-doubling perimetry. Invest Ophthalmol Vis Sci 1997; 38: 413–425.
Brusini P, Busatto P . Frequency doubling perimetry in glaucoma early diagnosis. Acta Ophthalmol Scand 1998; 76: S227: 23–24.
Quigley HA . Identification of glaucoma-related visual field abnormality with the screening protocol of frequency doubling technology. Am J Ophthalmol 1998; 125: 819–829.
Cello KE, Nelson-Quigg JM, Johnson CA . Frequency doubling technology perimetry for detection of glaucomatous visual field loss. Am J Ophthalmol 2000; 129: 314–322.
Kelly DH . Frequency doubling in visual responses. Opt Soc Am 1996; 56: 1628–1633.
Anderson AJ, Johnson CA . Mechanisms isolated by frequency-doubling technology perimetry. Invest Ophthalmol Vis Sci 2002; 43: 398–401.
White AJR, Sun H, Swanson WH, Lee BB . An examination of physiological mechanisms underlying the frequency-doubling illusion. Invest Ophthalmol Vis Sci 2002; 43: 3590–3599.
Sample PA, Bosworth CF, Blumenthal EZ, Girkin C, Weinreb RN . Visual function-specific perimetry for indirect comparison of different ganglion cell populations in glaucoma. Invest Ophthalmol Vis Sci 2000; 41: 783–790.
Bowd C, Zangwill LM, Berry CC, Blumenthal EZ, Vasile C, Sanchez-Galeana C et al. Detecting early glaucoma by assessment of retinal nerve fiber layer thickness and visual function. Invest Ophthalmol Vis Sci 2001; 42 (9): 1993–2003.
Paczka JA, Friedman DS, Quigley HA, Barron Y, Vitale S . Diagnostic capabilities of frequency-doubling technology, scanning laser polarimetry, and nerve fiber layer photographs to distinguish glaucomatous damage. Am J Ophthalmol 2001; 131: 188–197.
Spry PG, Johnson CA, Mansberger SL, Cioffi GA . Psychophysical investigation of ganglion cell loss in early glaucoma. J Glaucoma 2005; 14: 11–19.
Kondo Y, Yamamoto T, Sato Y, Matsubara M, Kitazawa Y . A frequency-doubling perimetric study in normal-tension glaucoma with hemifield defects. J Glaucoma 1998; 7: 261–265.
Bayer AU, Erb C . Short wavelength automated perimetry, frequency doubling technology perimetry and pattern electroretinography for prediction of progressive glaucomatous standard visual field defects. Ophthalmology 2002; 109: 1009–1017.
Landers JA, Goldberg I, Graham SL . Detection of early visual field loss in glaucoma using frequency-doubling perimetry and short-wavelength automated perimetry. Arch Ophthalmol 2003; 121: 1705–1710.
Medeiros FA, Sample PA, Weinreb RN . Frequency doubling technology perimetry abnormalities as predictors of glaucomatous visual field loss. Am J Ophthalmol 2004; 137: 863–871.
Kamantigue MEG, Joson PJ, Chen PP . Prediction of visual field defects on standard automated perimetry by screening C-20–1 frequency doubling technology perimetry. J Glaucoma 2006; 15: 35–39.
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W et al. Optical coherence tomography. Science 1991; 54: 1178–1181.
Schuman JS, Hee MR, Puliafito CA, Wong C, Pedut-Kloizman T, Lin CP et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol 1995; 113: 586–596.
Bowd C, Weinreb RN, Williams JM, Zangwill LM . The retinal nerve fiber layer thickness in ocular hypertensive, normal, and glaucomatous eyes with optical coherence tomography. Arch Ophthalmol 2000; 118: 22–26.
Zangwill LM, Bowd C, Berry CC, Williams J, Blumenthal EZ, Sanchez-Galeana CA et al. Discriminating between normal and glaucomatous eyes using the Heidelberg Retina Tomograph; GDx Nerve Fiber Analyzer, and Optical Coherence Tomograph. Arch Ophthalmol 2001; 119: 985–993.
El Beltagi TA, Bowd C, Boden C, Amini P, Sample PA, Zangwill LM et al. Retinal nerve fiber layer thickness measured with optical coherence tomography is related to visual function in glaucomatous eyes. Ophthalmology 2003; 110: 2185–2191.
Schuman JS, Wollstein G, Farra T, Hertzmark E, Aydin A, Fujimoto JG et al. Comparison of optic nerve head measurements obtained by optical coherence tomography and confocal scanning laser ophthalmoscopy. Am J Ophthalmol 2003; 135: 504–512.
Medeiros FA, Zangwill LM, Bowd C, Weinreb RN . Comparison of the GDx VCC Scanning Laser Polarimeter, HRT II Confocal Scanning Laser Ophthalmoscope, and Stratus OCT Optical Coherence Tomograph for the detection of glaucoma. Arch Ophthalmol 2004; 122: 827–837.
Budenz DL, Michael A, Chang RT, McSoley J, Katz J . Sensitivity and specificity of the Stratus for perimetric glaucoma. Ophthalmology 2005; 112: 3–9.
Medeiros FA, Zangwill LM, Bowd C, Vessani RM, Susanna Jr R, Weinreb RN . Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol 2005; 139: 44–55.
Wollstein G, Ishikawa H, Wang J, Beaton SA, Schuman JS . Comparison of three optical coherence tomography scanning areas for detection of glaucomatous damage. Am J Ophthalmol 2005; 139: 39–43.
Leung CK, Chan W, Chong KK-L, Yung W, Tang K, Woo J et al. Comparative study of retinal nerve fiber layer measurement by Stratus and GDx VCC, I: Correlation analysis in glaucoma. Invest Ophthalmol Vis Sci 2005; 46: 3214–3220.
Bourne RRA, Medeiros FA, Bowd C, Jahanbakhsh K, Zangwill LM, Weinreb RN . Comparability of retinal nerve fiber layer thickness measurements of optical coherence tomography instruments. Invest Ophthalmol Vis Sci 2005; 46: 1280–1285.
Mok KH, Lee VW, So KF . Retinal nerve fiber layer measurement by optical coherence tomography in glaucoma suspects with short-wavelength perimetry abnormalities. J Glaucoma 2003; 12: 45–49.
Doughty MJ, Zaman ML . Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol 2000; 44: 367–408.
Anderson D, Patella V . Automated Static Perimetry. St Louis, MO: Mosby, 1999, pp 117.
Medeiros FA, Sample PA, Weinreb RN . Corneal thickness measurements and frequency doubling technology perimetry abnormalities in ocular hypertensive eyes. Ophthalmology 2003; 110: 1903–1908.
Phelps CE, Hutson A . Estimating diagnostic test accuracy using a ‘fuzzy gold standard’. Med Decis Making 1995; 15: 44–57.
Wollsein G, Schuman JS, Price LL, Aydin A, Stark PC, Hertzmark E et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol 2005; 123: 464–470.
Nouri-Mahdavi K, Hoffman D, Tannenbaum DP, Law SK, Caprioli J . Identifying early glaucoma with optical coherence tomography. Am J Ophthalmol 2004; 137: 228–235.
Kanamori A, Nakamura M, Escano MF, Seya R, Maeda H, Negi A . Evaluation of the glaucomatous damage on retinal nerve fiber layer thickness measured by optical coherence tomography. Am J Ophthalmol 2003; 135: 513–520.
Parisi V, Manni G, Gandolfi SA, Centofanti M, Colacino G, Bucci MG . Visual function correlates with nerve fiber layer thickness in eyes affected by ocular hypertension. Invest Ophthalmol Vis Sci 1999; 40: 1828–1833.
Sanchez-Galeana CA, Bowd C, Zangwill LM, Sample PA, Weinreb RN . Short-wavelength automated perimetry results are correlated with optical coherence tomography retinal nerve fiber layer thickness measurements in glaucomatous eyes. Ophthalmology 2004; 111: 1866–1872.
Bagga H, Greenfield DS . Quantitative assessment of structural damage in eyes with localized visual filed abnormalities. Am J Ophthalmol 2004; 137: 797–805.
Mistlberger A, Liebmann M, Greenfield DS, Hoh ST, Ishikawa H, Marmor M et al. Assessment of optic disc anatomy and nerve fiber layer thickness in ocular hypertensive subjects with normal short-wavelength automated perimetry. Ophthalmology 2002; 109: 1362–1366.
Brandt D, Beiser JA, Kass MA, Gordon MO, the Ocular Hypertension Treatment Study (OHTS) Group. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS). Ophthalmology 2001; 108: 1779–1788.
Zeppieri M, Brusini P, Miglior S . Corneal thickness and functional damage in patients with ocular hypertension. Eur J Ophthalmol 2005; 15: 196–201.
Acknowledgements
Ethics approval: This study was approved by the Ethics Committee of the S Maria della Misericordia Hospital, Udine, Italy. Financial disclosure: The authors have no financial interest in any devices used in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brusini, P., Zeppieri, M., Tosoni, C. et al. Stratus-OCT imaging in early glaucomatous and in ocular hypertensive patients with and without frequency-doubling technology abnormalities. Eye 22, 406–413 (2008). https://doi.org/10.1038/sj.eye.6702654
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702654