Abstract
Id protein family consists of four members namely Id-1 to Id-4. Different from other basic helix–loop–helix transcription factors, they lack the DNA binding domain. Id proteins have been shown to be dysregulated in many different cancer types and their prognostic value has also been demonstrated. Recently, Id-1 has been shown to be upregulated in oesophageal squamous cell carcinoma (ESCC). However, the prognostic implications of Id proteins in ESCC have not been reported. We examined the expression of the Id proteins in ESCC cell lines and clinical ESCC specimens and found that Id protein expressions were dysregulated in both the ESCC cell lines and specimens. By correlating the expression levels of Id proteins and the clinicopathological data of our patient cohort, we found that M1 stage tumours had significantly higher nuclear Id-1 expression (P=0.012) while high nuclear Id-1 expression could predict development of distant metastasis within 1 year of oesophagectomy (P=0.005). In addition, high levels of Id-2 expression in both cytoplasmic and nuclear regions predicted longer patient survival (P=0.041). Multivariate analysis showed that high-level expression of Id-2 in both cytoplasmic and nuclear regions and lower level of nuclear Id-1 expression were independent favourable predictors of survival in our ESCC patients. Our results suggest that Id-1 may promote distant metastasis in ESCC, and both Id-1 and Id-2 may be used for prognostication for ESCC patients.
Similar content being viewed by others
Main
Oesophageal cancer is the eighth most common cancer and the sixth most common cause of cancer-related death worldwide with a 20-fold higher risk being observed in China when compared to other low-risk areas (Parkin et al, 2005). While the incidence of oesophageal adenocarcinoma in western countries is increasing, the predominant type of oesophageal cancer is still squamous cell carcinoma (Lam, 2000).
Id protein family is a group of helix–loop–helix transcription factors that have been implicated in different steps in carcinogenesis such as tumorigenesis, differentiation and metastasis (Benezra et al, 2001; Zebedee and Hara, 2001; Hasskarl and Munger, 2002; Sikder et al, 2003; Fong et al, 2004; Wong et al, 2004). Recent studies exploring the potential use of Id proteins as tumour markers have revealed that they play different roles in different types of cancer such that they might act as markers for progression, metastasis and prognosis in some cancer types. For instance, a high-level expression of Id-1 has been shown to be associated with poor prognosis of early-stage cervical cancer (Schindl et al, 2001), node-negative breast cancer (Schoppmann et al, 2003) and malignant melanoma (Straume and Akslen, 2005). Id-1 has also been shown to promote metastasis through increasing invasiveness and angiogenesis in breast and prostate cancer (Fong et al, 2003; Ling et al, 2005). High level of Id-2 has been demonstrated to reduce breast cancer cells invasiveness and therefore could act as a favourable prognostic marker for breast cancer patients (Stighall et al, 2005). In addition, the methylation status of Id-4 has been shown to correlate with poor prognosis in colorectal cancer (Umetani et al, 2004). Taken together, these results suggest that Id proteins are important in cancer initiation and progression, and that they may be useful prognostic markers.
Id-1 has been shown to be overexpressed in oesophageal squamous cell carcinoma (ESCC) (Hu et al, 2001). A recent study on functional roles of Id-1 in ESCC revealed that expression of Id-1 increased proliferation and decreased TNF-α-induced apoptosis in ESCC cell lines (Hui et al, 2006). These results suggest that Id-1 plays an important role in tumorigenesis of ESCC. However, the prognostic significance of Id-1 has not been adequately studied, and the expressions of other members of Id protein family in ESCC remain to be elucidated.
Materials and methods
Patients and specimens
The patient cohort consists of 84 ESCC Chinese patients who had undergone total oesophagectomy for ESCC in Queen Mary Hospital from 1998 to 2005. The clinical data collected prospectively are summarised in Table 1. Briefly, the patients recruited to this study had not received any chemoradiotherapy directed against ESCC prior to oesophagectomy. The oesophagectomy specimens were processed for routine histopathologic diagnosis. Tissue blocks were fixed in 10% buffered formalin overnight and then processed for paraffin embedding. Non-neoplastic oesophageal epithelium was selected from the upper resection margin of the oesophagectomy specimens. Clinical stage at the time of operation was classified according to the TNM staging system, while tumour grades were classified based on the World Health Organization classification (Hamilton and Aaltonen, 2000). Patients who developed distant metastasis within 1 year of oesophagectomy were regarded to have ESCC of high metastatic potential, while those who did not were considered to have ESCC of low metastatic potential. The median follow-up of our patient cohort is 12.6 months (ranged from 0.7 to 65.1 months).
Cell lines
Immortalise oesophageal epithelial cell line NE1 was maintained in KSFM (Invitrogen, Carlsbad, CA, USA). ESCC cell lines EC18, EC109 and KYSE70 were maintained in RPMI-1640 (Invitrogen) with 10% FBS. HKESC2 was maintained in MEM (Invitrogen) with 20% FBS. KYSE30 and KYSE150 were maintained in 1 : 1 RPMI-1640/Ham's F12 medium with 10 and 2% FBS, respectively.
Western blotting
Western blotting was performed as previously described (Kwok et al, 2005) using primary antibodies against Id-1 and Id-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a concentration of 1 : 500 and 1 : 200, respectively. Actin was used as a loading control by using anti-actin antibody (Sigma, St Louis, MO, USA).
Tissue microarray construction
Tissue microarray (TMA) was constructed with a Beecher Instruments tissue microarraryer (Beecher Instruments, Silver Spring, MD, USA). Three representative areas of each specimen, including 33 non-neoplastic oesophageal epithelium and 84 ESCC specimens, were selected by a pathologist (K-W Chan) and transferred to a TMA block using a 0.6 mm core diameter needle. Haematoxylin- and eosin-stained sections from each TMA block were checked by K-W Chan to ensure that adequate targeted areas had been included. The sample numbers varied slightly among staining results due to a variation in the number of interpretable specimens on TMA sections.
Immunohistochemical staining
Immunohistochemical staining using Dako EnVision+ system-HRP (Dako Corporation, Glostrup, Denmark) was carried out according to the manufacturer's instructions. Polyclonal anti-Id-1 (C-20), Id-2 (C-20), Id-3 (C-20) and Id-4 (H-70) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were applied at a 1 : 600, 1 : 800, 1 : 200 and 1 : 50 dilution, respectively, for overnight incubation at 4°C. For peptide-blocking analysis used to verify antibody specificity, five-fold (w/w) excess of blocking peptide (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was preincubated with the corresponding primary antibody overnight at 4°C before applying them to the TMA slides.
Evaluation of immunohistochemical staining results
The stained sections were reviewed by two independent observers (K-W Chan and Y-P Chan) who had no prior knowledge of the clincopathological data of the patient cohort. The ‘hot spot’ method was employed in evaluation of the staining results. All three cores of each specimen in the TMA were individually scored, and the highest score was selected for later statistical analysis. The extent and intensity of cytoplasmic staining was graded by an arbitrary scale ranged from 0 to 3, representing negative (0), weak (1), moderate (2) and strong (3) staining, respectively. Negative and weak cytoplasmic staining was classified as low expression, while moderate and strong cytoplasmic staining was classified as high expression. Nuclear staining was scored by the percentage of nuclei positively stained in the whole core with at least 200 nuclei counted. Specimens with nuclear staining score lower than the mean percentage were classified as low expression and vice versa. An overall grade of expression was then assigned by combining both cytoplasmic and nuclear staining results such that only specimens having both high cytoplasmic and nuclear staining scores were graded as high overall staining. Staining patterns other than that were classified as low overall expression.
Statistical analysis
Statistical analysis was performed using SPSS 14.0 software. Differences in expression level among different clinicopathological stages were analysed by χ2 test, Mann–Whitney U-test or Fisher's exact test where applicable. The association between expression level and the risk to develop distant metastasis within 1 year of oesophagectomy was estimated by odds ratio and 95% confidence intervals using binary logistic regression analysis. Survival curves between low- and high-level expressions of the four Id proteins were estimated by Kaplan–Meier analysis and compared by log-rank test. Cox regression analysis was performed to identify independent factors that predict patient survival. All factors with P-value <0.10 in the univariate analysis were allowed entry to the multivariate analysis. Backward stepwise selection was used with removal limits of P-value <0.05. A P-value <0.05 was considered significant in all the statistical analyses.
Results
Expression of Id-1 and Id-2 in oesophageal cell lines
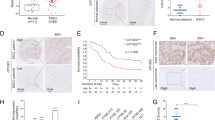
As shown in Figure 1, Id-1 was detected in four out of six ESCC cell lines with a high expression in three cell lines investigated. Id-2 was expressed in all six ESCC cell lines and was barely detected in NE1. These results show that Id-1 and Id-2 are dysregulated in ESCC as compared to immortalised oesophageal cell line NE1. In contrast, we found that Id-1 expression was slightly reduced when the cells were serum-starved. This result suggests that Id-1 expression in ESCC is only slightly affected by the presence or absence of serum.
Western blot analysis of Id-1 and Id-2 expression in immortalized oesophageal epithelial and ESCC cells. Cancer cells (2 × 105) were seeded in six-well plate with supplement of serum, according to the recommendation from the suppliers of the cell lines, as described in Materials and Methods. Medium was changed after 1 day with either serum-free medium or fresh serum-supplemented medium NE1 was cultured in KSFM with growth factors supplementation, and fresh medium was replaced after 1 day. Cells were harvested after 24 h. Protein was extracted and the expression level of Id-1 and Id-2 was then compared by Western blot analysis. Note that the expression of Id-1 was detected in four out of six ESCC cell lines while Id-2 was expressed in all ESCC cell lines tested. (B) The expression level of Id-1 in three ESCC cancer cell lines with or without serum supplementation. Id-1 expression level was slightly reduced when the cells were serum-starved for 24 h.
Id protein expressions in ESCC and non-neoplastic oesophageal epithelium specimens
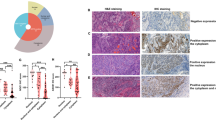
As shown in Figure 2, Id protein expressions were dysregulated in human ESCC specimens when compared to their non-neoplastic counterparts. Non-neoplastic oesophageal epithelium expressed a lower level of Id-1 than the tumour specimens. A majority (85%, 28 out of 33) of non-neoplastic oesophageal epithelium specimens showed negative cytoplasmic and nuclear Id-1 staining, while 36% (29 out of 80) and 29% (23 out of 80) of ESCC specimens showed moderate to strong cytoplasmic Id-1 staining and positive nuclear Id-1 staining, respectively. Both nuclear and cytoplasmic Id-2 expressions were relatively low in non-neoplastic oesophageal epithelium with 53% (17 out of 32) of specimens showed negative to weak staining. However, as many as 87% (69 out of 79) of ESCC specimens showed moderate to strong cytoplasmic Id-2 staining and 66% (52 out of 79) showed positive nuclear Id-2 staining. These results suggest that Id-1 and Id-2 were differentially expressed in non-neoplastic oesophageal epithelium and ESCC specimens. In addition, overexpression of Id-1 and Id-2 might be important in neoplastic transformation of ESCC.
Representative results of immunohistochemistry of Id-1 to Id-4 expression in oesophageal specimens. (Panel 1) Id-1 expression was negative to weak in non-neoplastic oesophageal epithelium (A), but increased in ESCC specimens (B–E). While a low level of nuclear Id-1 staining was detected in primary ESCC at M0 stage (B), a significantly higher level of nuclear Id-1 staining was observed in tumours at M1 stage (C). ESCC at T2 stage (D) had weak cytoplasmic Id-1 staining while a high-level cytoplasmic Id-1 expression was detected in T4 stage tumour (E). (Panel 2) Id-2 staining was relatively weak in normal oesophageal epithelium (F) while was higher in tumour specimens (G and H). High level of cytoplasmic Id-2 staining was observed in well-differentiated tumours (G) but was significantly lower in poorly differentiated tumours (H). (Panel 3) Id-3 staining was high in both normal oesophageal epithelium (I) and ESCC specimens (J), with mainly nuclear staining in the former one and both cytoplasmic and nuclear staining in the later one. (Panel 4) Id-4 expression was relatively weak in normal oesophageal epithelium (K) and higher in tumour specimens (L). Image was captured at × 400 magnification.
In contrast, expressions of Id-3 and Id-4 did not differ much between non-neoplastic epithelium and tumour tissues. Id-3 was positively detected in 94% (31 out of 33) of non-neoplastic specimens and in 90% (72 out of 80) of ESCC specimens. While 17 out of 33 (52%) non-neoplastic oesophageal epithelium samples expressed a low level of Id-4, 36 out of 80 (45%) ESCC specimens showed similar low expression. Id-3 was mainly expressed in the nucleus and sometimes in cytoplasm of non-neoplastic oesophageal epithelium, but its expression in ESCC was generally both cytoplasmic and nuclear. Id-4 was mainly detected in the nucleus of non-neoplastic oesophageal epithelium samples, while its expression in cytoplasm is more frequent in ESCC.
Id protein expressions correlated with clinicopathological parameters
As shown in Figures 2 and 3, we found that primary ESCC at M1 stage had significantly higher (P=0.012) nuclear expression of Id-1 (16.13±7.82%) than those at M0 stage (4.76±1.55%). Binary logistic regression was then employed to estimate the odds ratio for patients having high-level nuclear expression of Id-1 in their primary ESCC to develop distant metastasis within 1 year. Primary ESCC tumours with more than 11.86% (mean percentage of nuclei stained positive in ESCC specimens) of nuclei stained positive for Id-1 were regarded as having high and others as having low nuclear expression. The results showed that patients with high-level nuclear expression of Id-1 in their primary ESCC were at a higher risk to develop distant metastasis within 1 year (odds ratio: 4.615, 95% confidence interval (CI): 1.596−13.348, P=0.005).
In contrast, we observed that 59% of primary ESCC at the T4 stage showed a high cytoplasmic Id-1 expression, whereas only 30% of ESCC at the lower T stages with similar staining extent (Figure 4). By statistical analysis, we demonstrated that high-level cytoplasmic Id-1 expression was significantly (P=0.045) associated with T4 tumour.
Cytoplasmic expression of Id proteins in ESCC of different T stages. Significantly higher percentage of patients with T4 stage tumour had a higher cytoplasmic Id-1 expression when compared to patients with T1–T3 stages tumour (P=0.045). No significant association was found between cytoplasmic Id-2, -3 and -4 expressions and T stage.
In addition, a statistically significant association was observed between low-level cytoplasmic Id-2 expression and poor differentiation of the tumour (P=0.013). While 30% of poorly differentiated ESCC expressed low-level cytoplasmic Id-2, the same was observed in only 7% of well or moderately differentiated tumours (Figure 5). However, expression levels of both Id-3 and Id-4 were not significantly associated with any of the clincopathological parameters analysed. These results suggest that dysregulations of Id-1 and Id-2 are involved in tumour progression and metastasis of ESCC.
Cytoplasmic expression of Id proteins in ESCC of different histological grades. Significantly higher percentage of patients with poorly differentiated tumour had a low cytoplasmic Id-2 expression when compared to patients with well or moderately differentiated tumour (P=0.013). No significant association was found between cytoplasmic Id-1, -3 and -4 expression and histological grade.
To investigate the specificity of Id-1 and Id-2 antibodies in immunohistochemistry, we performed a peptide neutralisation analysis. By preincubating the primary antibody with five-fold (w/w) excess of corresponding blocking peptide, the signal was almost completely abolished (Figure 6). These results confirmed the specificity of the antibodies used.
Id-1 and Id-2 are independent prognostic factors for survival in our ESCC cohorts
By Kaplan–Meier analysis, a high overall grade combining cytoplasmic and nuclear expression of Id-2 significantly (P=0.041) predicted better survival in our cohort (Figure 7). The mean survival was 28.9 (95% CI: 16.7−41.1) months for patients with high overall Id-2 expression and 16.8 (95% CI: 12.3−21.1) months for those with low overall Id-2 expression. To identify independent predictors for survival, univariate and multivariate Cox-regression analyses were performed. All the clinicopathological parameters and Id protein expression levels including age, sex, TNM stage, histological stage, and cytoplasmic, nuclear and overall Id protein expressions were entered into univariate Cox-regression analysis. We found that only T4 stage (P=0.007), M1 stage (P=0.026) and low overall Id-2 expression (P=0.045) were significant predictors of poor survival in univariate analysis (Table 2). Factors with P⩽0.10 in univariate analysis were allowed entry to multivariate Cox-regression analysis. We found that increasing age (P=0.017), male sex (P=0.019), increasing nuclear Id-1 expression (P=0.011) and low overall Id-2 expression (P=0.020) individually contributed significantly to poor prognosis as shown by the multivariate analysis (Table 2).
Discussion
Id proteins have been recently demonstrated to promote cancer progression and metastasis and are potential prognostic factors in different types of cancer. In this study, we aimed to investigate how this knowledge could be applied to further our understanding of the pathogenesis and progression of ESCC. The expression levels of Id proteins in ESCC and non-neoplastic oesophageal epithelium in corresponding oesophagectomy specimens were studied by immunohistochemistry and the results were correlated with clinicopathological parameters. We observed that primary tumours at M1 stage had a significantly higher nuclear Id-1 expression than M0 stage tumours (P=0.012). A high level of cytoplasmic Id-1 expression was significantly associated with T4 stage tumour (P=0.045). In contrast, low-level cytoplasmic Id-2 expression was significantly associated with poorly differentiated tumours (P=0.013). In addition, high level of overall Id-2 expression in primary tumour predicted better survival of ESCC patient in Kaplan–Meier analysis (P=0.041). By multivariate Cox-regression analysis, we were able to demonstrate that increasing nuclear Id-1 expression (P=0.011) and low overall Id-2 expression (P=0.020) were independent predictors of poor overall survival in our ESCC patients.
Id-1 has been shown to promote metastasis in several different types of cancer. Overexpression of Id-1 promotes mammary epithelial cell invasion (Desprez et al, 1998), and reduced expression of Id-1 in metastatic breast cancer cells leads to a decrease in invasiveness in vitro (Fong et al, 2003). Overexpression of Id-1 in prostate cancer cells also promotes metastasis through increased angiogenesis (Ling et al, 2005) and invasiveness (Coppe et al, 2004). Reduced expressions of Id-1 and Id-3 have been demonstrated to inhibit the metastatic ability of gastric cancer cells (Tsuchiya et al, 2005), and Id-1 expression has been shown to be associated with the presence of invasion in endometrial carcinoma (Takai et al, 2001) and tumour angiogenesis in human pancreatic cancer (Lee et al, 2004). In lines with these results, we found that primary ESCC at M1 stage had a significantly (P=0.012) higher nuclear expression of Id-1 than the M0 stage tumours. In addition, by binary logistic regression, we observed that a high nuclear expression of Id-1 in primary ESCC tumours significantly predicted the development of distant metastasis of the corresponding patients within 1 year of oesophagectomy (odds ratio: 4.615, 95% CI: 1.596–13.348, P=0.005). In addition, a high level of cytoplasmic Id-1 expression was significantly (P=0.045) correlated with T4 stage tumours. These results suggest that upregulation of Id-1 in ESCC might promote distant metastasis and cancer progression. In vitro experiments are being undertaken to investigate the functional role of Id-1 in metastatic progression of ESCC.
Id-2 has been suggested to promote cellular differentiation in normal murine mammary epithelial cells (Parrinello et al, 2001). In breast cancer, Id-2 expressed at high levels maintains the cancer cells in a non-aggressive phenotype (Itahana et al, 2003). In addition, high level of Id-2 expression has been shown to reduce the invasiveness of breast cancer cells and can act clinically as a favourable prognostic marker (Stighall et al, 2005). Similar results have been obtained in intestinal epithelium where Id-2 promotes differentiation and inhibit tumorigenesis (Russell et al, 2004). Our results on Id-2 expression in ESCC also showed that low level of cytoplasmic Id-2 expression was significantly (P=0.013) associated with poor tumour differentiation. Moreover, high level of both nuclear and cytoplasmic expression of Id-2 provides favourable prognosis for our ESCC patients, with mean survival of 28.9 (95% CI: 16.7–41.1) months when compared to those having low overall Id-2 expression (16.8 (95% CI: 12.3–21.1) months).
In the present study, we observed that Id-2 expression was low in the immortalised oesophageal cell line NE1 and the non-neoplastic oesophageal epithelium samples showed lower Id-2 expression than their cancer counterparts. These results suggested that Id-2 promotes neoplastic transformation of oesophageal epithelial cells. Id-2 has been shown to bind and inhibit the function of retinoblastoma protein, a tumour suppressor protein (Iavarone et al, 1994; Lasorella et al, 1996) and therefore has been implicated in tumour progression in several cancer types (Maruyama et al, 1999; Fukuma et al, 2003; Coppe et al, 2004). We speculated that overexpression of Id-2 might promote neoplastic transformation of oesophageal epithelium through destruction of the normal cell cycle checkpoint and increased proliferation. After onset of other oncogenic events, reduced Id-2 expression might increase aggressiveness of ESCC cancer cells through dedifferentiation and leads to poor patient survival.
To test whether Id-1 and Id-2 might act as independent prognostic factors in our patient cohort, Cox-regression with backward stepwise selection was carried out. The results (Table 2) showed that higher nuclear expression of Id-1 (P=0.011) and low-level overall expressions of Id-2 (P=0.020) were independent predictors for poor prognosis in our cohort of ESCC patients.
In summary, our results suggest that overexpression of Id-1 can promote neoplastic transformation of normal oesophageal epithelium, ESCC progression and the development of distant metastasis. In contrast, expression of Id-2 might inhibit the emergence of a more aggressive phenotype of ESCC. We provide new evidence that Id-1 and Id-2 may be useful prognostic indicators of metastatic potential of ESCC and survival of patients, respectively.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Benezra R, Rafii S, Lyden D (2001) The Id proteins and angiogenesis. Oncogene 20: 8334–8341
Coppe J, Itahana Y, Moore D, Bennington J, Desprez P (2004) Id-1 and Id-2 proteins as molecular markers for human prostate cancer progression. Clin Cancer Res 10: 2044–2051
Desprez P, Lin C, Thomasset N, Sympson C, Bissell M, Campisi J (1998) A novel pathway for mammary epithelial cell invasion induced by the helix–loop–helix protein Id-1. Mol Cell Biol 18: 4577–4588
Fong S, Debs R, Desprez P (2004) Id genes and proteins as promising targets in cancer therapy. Trends Mol Med 10: 387
Fong S, Itahana Y, Sumida T, Singh J, Coppe J, Liu Y, Richards P, Bennington J, Lee N, Debs R, Desprez P (2003) Id-1 as a molecular target in therapy for breast cancer cell invasion and metastasis. Proc Natl Acad Sci USA 100: 13543–13548
Fukuma M, Okita H, Hata J, Umezawa A (2003) Upregulation of Id2, an oncogenic helix–loop–helix protein, is mediated by the chimeric EWS/ets protein in Ewing sarcoma. Oncogene 22: 1–9
Hamilton S, Aaltonen L (2000) World Health Organization Classification of Tumors. Pathology and Genetics of Tumors of the Digestive System. Lyon: IARC
Hasskarl J, Munger K (2002) Id proteins – tumor markers or oncogenes? Cancer Biol Ther 1: 91–96
Hu Y, Lam K, Law S, Wong J, Srivastava G (2001) Identification of differentially expressed genes in esophageal squamous cell carcinoma (ESCC) by cDNA expression array: overexpression of Fra-1, Neogenin, Id-1, and CDC25B genes in ESCC. Clin Cancer Res 7: 2213–2221
Hui C, Cheung P, Ling M, Tsao S, Wang X, Wong Y, Cheung A (2006) Id-1 promotes proliferation of p53-deficient esophageal cancer cells. Int J Cancer 119: 508–514
Iavarone A, Garg P, Lasorella A, Hsu J, Israel M (1994) The helix–loop–helix protein Id-2 enhances cell proliferation and binds to the retinoblastoma protein. Genes Dev 8: 1270–1284
Itahana Y, Singh J, Sumida T, Coppe J, Parrinello S, Bennington J, Desprez P (2003) Role of Id-2 in the maintenance of a differentiated and noninvasive phenotype in breast cancer cells. Cancer Res 63: 7098–7105
Kwok W, Ling M, Lee T, Lau T, Zhou C, Zhang X, Chua C, Chan K, Chan F, Glackin C, Wong Y, Wang X (2005) Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res 65: 5153–5162
Lam A (2000) Molecular biology of esophageal squamous cell carcinoma. Crit Rev Oncol Hematol 33: 71–90
Lasorella A, Iavarone A, Israel M (1996) Id2 specifically alters regulation of the cell cycle by tumor suppressor proteins. Mol Cell Biol 16: 2570–2578
Lee K, Lee Y, Lee J, Choi S, Rhee J, Paik S, Kong G (2004) Overexpression of Id-1 is significantly associated with tumour angiogenesis in human pancreas cancers. Br J Cancer 90: 1198–1203
Ling M, Lau T, Zhou C, Chua C, Kwok W, Wang Q, Wang X, Wong Y (2005) Overexpression of Id-1 in prostate cancer cells promotes angiogenesis through the activation of vascular endothelial growth factor (VEGF). Carcinogenesis 26: 1668–1676
Maruyama H, Kleeff J, Wildi S, Friess H, Buchler M, Israel M, Korc M (1999) Id-1 and Id-2 are overexpressed in pancreatic cancer and in dysplastic lesions in chronic pancreatitis. Am J Pathol 155: 815–822
Parkin D, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108
Parrinello S, Lin C, Murata K, Itahana Y, Singh J, Krtolica A, Campisi J, Desprez P (2001) Id-1, ITF-2, and Id-2 comprise a network of helix–loop–helix proteins that regulate mammary epithelial cell proliferation, differentiation, and apoptosis. J Biol Chem 276: 39213–39219
Russell R, Lasorella A, Dettin L, Iavarone A (2004) Id2 drives differentiation and suppresses tumor formation in the intestinal epithelium. Cancer Res 64: 7220–7225
Schindl M, Oberhuber G, Obermair A, Schoppmann S, Karner B, Birner P (2001) Overexpression of Id-1 protein is a marker for unfavorable prognosis in early-stage cervical cancer. Cancer Res 61: 5703–5706
Schoppmann S, Schindl M, Bayer G, Aumayr K, Dienes J, Horvat R, Rudas M, Gnant M, Jakesz R, Birner P (2003) Overexpression of Id-1 is associated with poor clinical outcome in node negative breast cancer. Int J Cancer 104: 677–682
Sikder H, Devlin M, Dunlap S, Ryu B, Alani R (2003) Id proteins in cell growth and tumorigenesis. Cancer Cell 3: 525–530
Stighall M, Manetopoulos C, Axelson H, Landberg G (2005) High ID2 protein expression correlates with a favourable prognosis in patients with primary breast cancer and reduces cellular invasiveness of breast cancer cells. Int J Cancer 115: 403–411
Straume O, Akslen L (2005) Strong expression of ID1 protein is associated with decreased survival, increased expression of ephrin-A1/EPHA2, and reduced thrombospondin-1 in malignant melanoma. Br J Cancer 93: 933–938
Takai N, Miyazaki T, Fujisawa K, Nasu K, Miyakawa I (2001) Id1 expression is associated with histological grade and invasive behavior in endometrial carcinoma. Cancer Lett 165: 185–193
Tsuchiya T, Okaji Y, Tsuno N, Sakurai D, Tsuchiya N, Kawai K, Yazawa K, Asakage M, Yamada J, Yoneyama S, Kitayama J, Osada T, Watanabe T, Tokunaga K, Takahashi K, Nagawa H (2005) Targeting Id1 and Id3 inhibits peritoneal metastasis of gastric cancer. Cancer Sci 96: 784–790
Umetani N, Takeuchi H, Fujimoto A, Shinozaki M, Bilchik A, Hoon D (2004) Epigenetic inactivation of ID4 in colorectal carcinomas correlates with poor differentiation and unfavorable prognosis. Clin Cancer Res 10: 7475–7483
Wong Y, Wang X, Ling M (2004) Id-1 expression and cell survival. Apoptosis 9: 279–289
Zebedee Z, Hara E (2001) Id proteins in cell cycle control and cellular senescence. Oncogene 20: 8317–8325
Acknowledgements
This work was supported by a CRGC Research Grant (no. 200607176035) of The University of Hong Kong to K-W Chan.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Yuen, HF., Chan, YP., Chan, KK. et al. Id-1 and Id-2 are markers for metastasis and prognosis in oesophageal squamous cell carcinoma. Br J Cancer 97, 1409–1415 (2007). https://doi.org/10.1038/sj.bjc.6604035
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604035
Keywords
This article is cited by
-
High expression of ID1 in monocytes is strongly associated with phenotypic and functional MDSC markers in advanced melanoma
Cancer Immunology, Immunotherapy (2020)
-
Cancer cell-secreted IGF2 instigates fibroblasts and bone marrow-derived vascular progenitor cells to promote cancer progression
Nature Communications (2017)
-
RhoGDI2 up-regulates P-glycoprotein expression via Rac1 in gastric cancer cells
Cancer Cell International (2015)
-
TGF-β1-induced expression of Id-1 is associated with tumor progression in gastric cancer
Medical Oncology (2014)
-
Targeting Id1 reduces proliferation and invasion in aggressive human salivary gland cancer cells
BMC Cancer (2013)