Abstract
It is known that interferon-γ (IFN-γ) is produced by activated T and NK lymphoid cells, mononuclear cells, and macrophage and dendritic cells. Our previous studies have shown that IFN-γ-like immunoreactivity also appears in human adrenal cortical tumour and phaeochromocytoma. To investigate whether human tumour cells can produce IFN-γ, we examined 429 biopsy specimens of 30 kinds of tumour and tumour-surrounding tissues in adrenal glands and in kidneys by using immunohistochemistry and in situ hybridisation. IFN-γ immunoactivity was shown in 34.3% of the adrenal cortical adenomas, 50% of the adrenal cortical carcinomas, 26.7% of the phaeochromocytomas, 26.7% of the clear cell renal cell carcinomas (RCCs), 22% of the adrenal cortexes and 40% of medullas adjacent to tumours. The positive samples and expression areas were well overlapped between the IFN-γ mRNA and the immunohistochemistry staining. Western blot analysis has further confirmed the immunohistochemistry results by showing a distinct IFN-γ band corresponding to 17.4 kDa in tissue extracts from adrenal cortical adenoma, phaeochromocytoma and clear cell RCCs. These results indicate that IFN-γ is produced by some types of tumour cells, suggesting it may play a dual role in the development of these tumours.
Similar content being viewed by others
Main
Interferon-γ (IFN-γ) is a multi-functional cytokine produced mainly by macrophages, dendritic cells, activated T lymphocytes and NK cells (Tripp et al, 1993; Gessani and Belardelli, 1998; Fukao et al, 2000; Murphy et al, 2000). In addition to its well-known antiviral activities, IFN-γ also plays a role in host antitumor responses. Endogenously produced IFN-γ is reported to function in cancer immunosurveillance, protecting the host against the growth of transplanted tumours and the formation of primary, chemically induced spontaneous tumours (Shankaran et al, 2001; Street et al, 2001). Mice deficiency of IFN-γ signalling developing spontaneous tumours at a higher frequency than normal mice further indicates that IFN-γ is involved in immune response to tumours (Kaplan et al, 1998). IFN-γ also regulates cell proliferation and apoptosis via activating the downstream JAK–STAT signalling pathway (Buard et al, 1998; Haque and Williams, 1998; Weber-Nordt et al, 1998; Lee et al, 2000). IFN-γ-like immunoreactivity has been proved to appear in human adrenal cortical tumours, phaeochromocytomas and hepatocellular carcinomas (HCCs) by immunohistochemical method (Li and Song, 1995, 1996; Chia et al, 2002). However, immunohistochemistry analysis alone cannot confirm whether tumour cells produce IFN-γ. To answer this question, we used immunohistochemistry combined with in situ hybridisation and Western blot method to screen a cluster of different types of tumours.
Materials and methods
Biopsy material
A total of 429 biopsy specimens of 30 kinds of tumour and three types of tumour-surrounding tissues were collected from patients who had not received any chemotherapeutic or immunomodulatory treatment before operations. Tumour tissues were classified according to the classification system of the World Health Organization (Table 1). Tissue specimens were fixed for 18–24 h in 10% buffered formalin and routinely processed to paraffin embedding.
Immunohistochemistry
Serial sections from each case were stained with a mouse monoclonal antibody specific to human IFN-γ (United States Patent 4599306, Department of Immunology, Fourth Military Medical University, Xi'an, China). This antibody has optimised specificity by using the method of microwave antigen retrieval on formalin-fixed, paraffin-embedded tissue. Immunohistochemical staining was performed using a standard streptavidin–biotin–peroxidase complex (SABC) (BOSTER Incorporation, Wuhan, China). Visualisation was performed using diaminobenzidine (DAB) for the horseradish peroxidase. The sections were counterstained with haematoxylin, dehydrated and mounted. A positive control was performed using the L26 (anti-CD20) monoclonal antibody (Dako, Glostrup, Denmark) a marker of B lymphocytes. Negative controls were obtained by replacing the primary antibody with non-immune mouse serum.
Immunoblotting
Fresh tissue samples from six cases of adrenal cortical adenomas, five cases of phaeochromocytomas, five cases of clear cell renal cell carcinomas (RCCs) and one case of colonic adenocarcinoma were cut into small pieces and homogenised using a Polytrope high-speed metal homogeniser (Brinkmann Instruments, Westbury, NY, USA) in five volumes of 50 mmol l−1 Tris buffer (pH 7.7) containing 0.5 mmol l−1 phenylmethylsulphonylfluoride to inhibit proteolysis. The supernatant was obtained after centrifugation at 12 000 r.p.m. min−1 for 15 min at 4°C. A volume of 1 μl supernatant from each sample was dot blotted on a polyvinylidene fluoride (PVDF) paper. Immunostaining was performed using a standard SABC (BOSTER Incorporation). Negative control was obtained by replacing the primary antibody with non-immune mouse serum.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blot analysis
The dot blotting positive samples (two from adenomas, one from phaeochromocytoma and one from clear cell RCCs) and one negative colonic carcinoma were separated by 15% SDS–polyacrylamide gels (PAGE). Molecular weight markers (C-6210; Sigma Chemical Co, St Louis, MO, USA) were run together with the samples. After the separation, the proteins were electroblotted onto PVDF paper utilising the Phast system. The PVDF membranes were blocked by incubation in 0.02 mol l−1 Na2HPO4, 0.15 mol l−1 NaCl, pH 7.2 containing 0.05% Tween 20 and 3% bovine serum albumin for 1 h at room temperature and then followed by immunoblotting. Negative control was obtained by replacing the primary antibody with non-immune mouse serum.
In situ hybridisation analysis
In situ hybridisation for IFN-γ mRNA was performed on further serial sections from 35 cases of adrenal cortical adenomas, four cases of adrenal cortical carcinomas, 30 cases of phaeochromocytomas, 60 cases of clear cell RCCs, 10 cases of HCCs, nine cases of tumour surrounding adrenal cortexes, five cases of medullas and 23 cases of tumour around renal tissues by using an oligonucleartide IFN-γ kit (BOSTER Incorporation). The experiment was performed according to the instruction. Briefly, after de-waxing and re-hydration, sections were pretreated with 0.3% hydrogen peroxide to suppress the endogenous peroxide. Tissues were postfixed with 1% paraformaldehyde and were further reacted with prehybridisation solution. A volume of 30 μl hybridisation solution was supplied on each tissue section with glass coverslips and incubated overnight at 38–42°C. Coverslips were removed and the sections were washed. After blocking solutions, the sections were incubated with biotinilated mouse-anti-digoxingin for 1 h at 37°C. The sections were then washed thoroughly and incubated with SABC at room temperature. Visualisation was performed by using DAB. The sections were counterstained with haematoxylin, then dehydrated and mounted.
Controls were processed with the omission of the probe and with mRNase before hybridisation.
Scoring of immunohistochemistry and in situ hybridisation
The immunoreactivity and in situ hybridisation were scored according to the staining extension and intensity. The intensity of staining was scored from 0 to 3: 0 for negative; 1 for weak; 2 for moderate; 3 for strong staining. The extension of immunoreactivity was estimated from 0 to 4: 0 for negative; 1 for 1–25%; 2 for 26–50%; 3 for 51–75%; 4 for 76–100% of positive immunoreactivity cells. The positive immunoreactivity or in situ hybridisation staining was determined by the combined staining score (intensity score plus extent score): 0 for ⩽2; 1 for 3; 2 for 4–5; 3 for 6–7.
Results
Immunohistochemistry
The immunoreactive IFN-γ-positive cells displayed distinct brown staining; immunoreactive materials appearing as densely packed granules in the entire or focal of cytoplasm, whereas immunostaining was undetectable in negative control sections, indicating that IFN-γ immunoreactive staining was specific.
Prominent staining cells for immunoreactive IFN-γ was observed in 12 out of 35 (34.3%) of the adrenal cortical adenomas, two of four (50%) of the adrenal cortical carcinomas, eight out of 30 (26.7%) of the phaeochromocytomas and 16 out of 60 (26.7%) of the clear cell RCC. The IFN-γ immunoreactivity was mainly restricted to tumour cells, and was also in some lymph cells and macrophages but was usually not in other non-tumour cells. The IFN-γ immunoreactivity was also demonstrated in two out of nine (22%) of the adrenal cortex and two out of five (40%) of the medulla tissues adjacent to tumours (Figures 1 and 2 and Table 2). However, IFN-γ immunoreactivity could not be detected in renal tissues surrounding clear cell RCCs. Surprisingly, the rest 260 tumour biopsies, originating from other organs than adrenal gland and kidney, did not show IFN-γ immunostaining in any single case.
IFN-γ immunoreactivity in (A) adrenal cortical adenoma, (B) cortical carcinoma, (C) phaeochromocytoma, (D) clear cell RCC, tumour adjacent tissue in (E) adrenal cortex (F) and medulla. The immunoreactive IFN-γ-positive cells show distinct brown staining. The immunoreactive materials often appear as densely packed granules filling the entire or focal of cytoplasm of tumour cells (arrow) and the interstitial tissue is negative (arrowhead). No IFN-γ immunoreactivity is seen in (G) kidney. Clear cell RCC (same case as in D) matched (H) negative control staining do not show any positive staining. Bar=50 μm.
In situ hybridisation for IFN-γ mRNA. The IFN-γ mRNA signals are seen in adrenal (A) cortical adenoma, (B) cortical carcinoma, (C) phaeochromocytoma, (D) clear cell RCC, tumour adjacent tissue (E) in adrenal cortex (F) and medulla. IFN-γ mRNA is apparent in the cytoplasm of parenchyma cells (arrow), not in interstitial tissue (arrowhead). IFN-γ mRNA signals are not seen in HCC (G). Adrenal cortical carcinoma (same case as in B) matched negative control with (H) mRNase do not show any positive staining. Bar=50 μm.
Western blot analysis
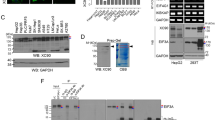
A distinct IFN-γ band corresponding to 17.4 kDa (Figure 3) was observed in tissue extracts from two out of six cases of the adrenal cortical adenomas, one out of five cases of the phaeochromocytomas and one out of five cases of the clear cell RCCs. Colonic adenocarcinoma and negative control did not show positive staining.
In situ hybridisation analysis
IFN-γ mRNA signals could only be seen in the sections reacted with antisense probe. It has no staining in control sections, which either have omitted IFN-γ probe or have prior incubated with mRNase. Prominent cytoplasmic staining for IFN-γ mRNA were detected in 13 out of 35 of the adrenal cortical adenomas, two out of four of the cortical carcinomas, nine out of 30 of the phaeochromocytomas, 16 out of 60 of the clear cell RCC, two out of nine of the tumour adjacent tissues in adrenal cortexes and two out of five of the medullas. No IFN-γ mRNA was detected in the renal tissues surrounding clear cell RCC. The IFN-γ expressing samples and areas were well overlapped in between of mRNA signal and immunohistochemistry staining (Figure 2).
Discussion
The adrenal gland consists of two distinct types of primary tumours: one arising from the adrenal cortex, including adenomas and carcinomas, and the other from the medulla representing neural crest-derived chromaffin cell tumours (pheaochromocytomas). RCC is a group of malignancies arising from the epithelium of the renal tubules. Clear cell RCC, one type of RCC, is composed of cells with clear or eosinophilic cytoplasm within a delicate vascular network.
Our previous study has demonstrated that IFN-γ-like immunoreactivity appeared in the cytoplasm of tumour cells in adrenal cortical adenoma, cortical carcinoma and pheochoromocytoma by using DB1 monoclonal antibody (Li and Song, 1995, 1996). The IFN-γ-like immunoreactivity is not a non-specific binding, since no staining was detected in the absence of primary antibody. In addition, DB1 monoclonal antibody is a high-affinity antibody against rat IFN-γ with specific labelling of different molecular forms of recombinant rat IFN-γ, inhibiting biological effects of IFN-γ in vitro and in vivo (Jacob et al, 1987; van der Meide et al, 1986). However, DB1 is a monoclonal antibody against rat IFN-γ but not human IFN-γ. In the present study, a monoclonal mouse-anti-human IFN-γ antibody was used to characterise the IFN-γ immunoactivity in 429 human biopsy samples from 30 different types of tumours and tumour-surrounding tissues in adrenal glands and in kidneys. The results showed that 34.3% of the adrenal cortical adenomas, 50% of the adrenal cortical carcinomas, 26.7% of the phaeochromocytomas and 26.7% of the clear cell RCC expressed IFN-γ immunoactivity. IFN-γ was not detectable in other types of tumour. Western blot analysis revealed IFN-γ band corresponding to 17.4 kDa in tissue extracts from two out of six cases of the adrenal cortical adenomas, one out of five cases of the phaeochromocytomas and one out of five cases of the clear cell RCCs, whereas the colonic adenocarcinoma and the negative control did not show any band. The results suggest that the IFN-γ immunoactivity is specific.
To confirm that the IFN-γ is indeed produced in tumour cells, IFN-γ mRNA in situ hybridisation was performed on the same biopsy samples. The in situ hybridisation results confirmed our hypothesis that the transcripts of IFN-γ genes were inside the adrenal and RCC tumour cells. The mRNA expressing tissues were overlapped with the immunohistochemistry staining, indicating that the IFN-γ protein was generated by tumour cells. IFN-γ protein and mRNA levels were generally higher in adrenal cortical adenomas than in cortical carcinomas. Clear cell RCCs expressed both mRNA and IFN-γ protein, but not in the tumour-surrounding renal tissue. Therefore, IFN-γ expression may in some way correlated to the tumour malignancy level or cell differentiation.
It remains unclear why IFN-γ was expressed in 22% of tumour surrounding tissue in adrenal cortexes and 40% of in medullas. Cells with IFN-γ-like immunoreactivity have also been reported in type II alveolar epithelial cells in interstitial lung disease (Wallace and Howie, 1999), rat neurons (Kiefer et al, 1991), rat skeletal muscle (Nennesmo et al, 1989), transplanted rat heart tissue (Wanders et al, 1992), astrocytes in active chronic multiple sclerosis lesion (Traugott and Lebon, 1988) and cerebrovascular endothelial cells from aged mice (Wei et al, 2000). However, there was no further report about the significance of the IFN-γ expression in those tissues or cells. IFN-γ has also been reported weakly expressed in HCCs (Chia et al, 2002). But in the present study, neither protein nor mRNA of IFN-γ was expressed in the 10 cases of HCCs, probably because that the methods we used are not sensitive enough.
It is well known that IFN-γ contributes to the increased antitumour activity of immune cells, though the reason why some tumour cells produce IFN-γ is not clear. Previous studies have established that testicular germ cell tumour cells can express both mRNA and protein of IFN-γ and the expressed IFN-γ is able to induce the chemokine IP-10, indicating its biological activity, whereas it is not able to phosphorylate the downstream STAT1 (Schweyer et al, 2002, 2003). A number of tumours and tumour cell lines have also been reported to develop a permanent and selective IFN-γ insensitivity, such as testicular germ cell tumours, RCC cell line, basal cell carcinoma of the skin and human lung adenocarcinoma cell lines (Kooy et al, 1998; Dovhey et al, 2000; Nagao et al, 2000; Schweyer et al, 2002, 2003; Dunn et al, 2005b). Such insensitivity to IFN-γ is due to lack of expression of IFN-γ receptors or the receptor downstream genes. Tumour cells can naturally develop mutations on IFN-γ downstream target genes, such as JAK and Stat1, to render them insensitive to IFN-γ.
Recently, a concept of cancer immunoediting has been put forward (Ikeda et al, 2002; Dunn et al, 2004a, 2004b, 2005a, 2005b, 2006). It is a process consisting of three phases: elimination (i.e., cancer immunosurveillance), equilibrium and escape. The immune system not only protects the host against development of primary nonviral cancers but also sculpts tumour immunogenicity. Thus, IFN-γ may play a dual role in preventing development of primary and transplanted tumours on one hand, and sculpting the immunogenic phenotype of tumours on the other. Tumour escape may be attributable to tumour genetic changes that affect tumour recognition by immune effective cells (such as, loss of antigen expression, loss of MHC components, shedding of NKG2D ligands and development of IFN-γ insensitivity) or provide tumours with mechanisms to escape immune destruction (such as, defects in death-receptor signalling pathways or expression of antiapoptotic signals including constitutively active STAT3). The dysregulation of MHC class I processing and presentation, or/and development of IFN-γ insensitivity in tumour cells, would allow tumours to escape from the elimination phase of the cancer immunoediting process (Dunn et al, 2004b). However, whether the specific expression of IFN-γ in tumours from adrenal gland and kidney is correlated with tumour escape is not clear and needs to be further elucidated.
In conclusion, we provide solid evidence that tumour cells express IFN-γ protein and mRNA especially in adrenal gland and kidney tumours. Our findings give a further insight into the role of IFN-γ in the pathogenesis of tumour, which will be critical for effective immunotherapeutic approaches for cancer therapy in the future.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Buard A, Vivo C, Monnet I, Boutin C, Pilatte Y, Jaurand MC (1998) Human malignant mesothelioma cell growth: activation of janus kinase 2 and signal transducer and activator of transcription 1alpha for inhibition by interferon-gamma. Cancer Res 58: 840–847
Chia CS, Ban K, Ithnin H, Singh H, Krishnan R, Mokhtar S, Malihan N, Seow HF (2002) Expression of interleukin-18, interferon-gamma and interleukin-10 in HCC. Immunol Lett 84: 163–172
Dovhey SE, Ghosh NS, Wright KL (2000) Loss of interferon-gamma inducibility of TAP1 and LMP2 in a renal cell carcinoma cell line. Cancer Res 60: 789–796
Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, Diamond MS, Koebel CM, Arthur C, White JM, Schreiber RD (2005a) A critical function for type I interferons in cancer immunoediting. Nat Immunol 6: 722–729
Dunn GP, Koebel CM, Schreiber RD (2006) Interferons, immunity and cancer immunoediting. Nat Rev Immunol 6: 836–848
Dunn GP, Old LJ, Schreiber RD (2004a) The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21: 137–148
Dunn GP, Old LJ, Schreiber RD (2004b) The three Es of cancer immunoediting. Annu Rev Immunol 22: 329–360
Dunn GP, Sheehan KC, Old LJ, Schreiber RD (2005b) IFN unresponsiveness in LNCaP cells due to the lack of JAK1 gene expression. Cancer Res 65: 3447–3453
Fukao T, Matsuda S, Koyasu S (2000) Synergistic effects of IL-4 and IL-18 on IL-12-dependent IFN-γ production by dendritic cells. J Immunol 164: 64–71
Gessani S, Belardelli F (1998) IFN-γ expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev 9: 117–123
Haque SJ, Williams BR (1998) Signal transduction in the interferon system. Semin Oncol 25: 14–22
Ikeda H, Old LJ, Schreiber RD (2002) The roles of IFN gamma in protection against tumour development and cancer immunoediting. Cytokine Growth Factor Rev 13: 95–109
Jacob OO, Van der Meide PH, McDevitt HO (1987) In vivo treatment of (NAB+NZW) F lupus-like nephritis with monoclonal antibody to γ interferon. J Exp Med 166: 798–803
Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD (1998) Demonstration of an interferon gamma-dependent tumour surveillance system in immunocompetent mice. Proc Natl Acad Sci USA 95: 7556–7561
Kiefer R, Haas CA, Kreutzberg GW (1991) Gamma interferon-like immunoreactive material in rat neurons: evidence against a close relationship to gamma interferon. Neuroscience 45: 551–560
Kooy AJ, Tank B, Vuzevski VD, van Joost T, Prens EP (1998) Expression of interferon-gamma receptors and interferon-gamma-induced up-regulation of intercellular adhesion molecule-1 in basal cell carcinoma; decreased expression of IFN-gamma R and shedding of ICAM-1 as a means to escape immune surveillance. J Pathol 184: 169–176
Lee CK, Smith E, Gimeno R, Gertner R, Levy DE (2000) STAT1 affects lymphocyte survival and proliferation partially independent of its role downstream of IFN-gamma. J Immunol 164: 1286–1292
Li Q, Song J (1995) Gamma interferon-like immunoreactivity in adrenal gland and cortical tumour. Zhonghua Binglixue Zazhi 24: 372–374
Li Q, Song J (1996) Expression of gamma interferon-like substance in adrenal pheochromocytoma. Zhonghuan Ne fen mi Za zhi 12: 90–92
Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy TL (2000) Signaling and transcription in T helper development. Annu Rev Immuno 18: 451–494
Nagao M, Nakajima Y, Kanehiro H, Hisanaga M, Aomatsu Y, Ko S, Tatekawa Y, Ikeda N, Kanokogi H, Urizono Y, Kobayashi T, Shibaji T, Kanamura T, Ogawa S, Nakano H (2000) The impact of interferon gamma receptor expression on the mechanism of escape from host immune surveillance in HCC. Hepatology 32: 491–500
Nennesmo I, Olsson T, Ljungdahl A, Kristensson K, Van der Meide PH (1989) Interferon-gamma-like immunoreactivity and T-cell marker expression in rat skeletal muscle fibres. Brain Rev 504: 306–310
Schweyer S, Soruri A, Baumhoer D, Peters J, Cattaruzza M, Radzun HJ, Fayyazi A (2002) Expression of CXC chemokine IP-10 in testicular germ cell tumours. J Pathol 197: 89–97
Schweyer S, Soruri A, Peters J, Wagner A, Radzun HJ, Fayyazi A (2003) A Malignant germ cell tumours of the testis express interferon-gamma, but are resistant to endogenous interferon-gamma. Br J Cancer 89: 915–921
Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD (2001) IFN gamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410: 1107–1111
Street SE, Cretney E, Smyth MJ (2001) Perforin and interferon-gamma activities independently control tumour initiation, growth, and metastasis. Blood 97: 192–197
Traugott U, Lebon PJ (1988) Interferon-gamma and Ia antigen are present on astroctes in active chronic multiple sclerosis lesion. J Neurol Sci 84: 257–264
Tripp CS, Wolf SF, Unanue ER (1993) Interleukin-12 and tumour necrosis factor are costimulators of interferon-γ production by natural killer cells in severe combined immuno-deficiency mice with listeriosis, and interleukin-10 is a physiologic ntagonist. Proc Natl Acad Sci USA 90: 3725–3729
van der Meide PH, Borman AH, Beljaars HG, Dubbeld MA, Botman CA, Schellekens H (1986) The purification and characterization of rat gamma interferon by use of two monoclonal antibodies. J Gen Virol 67: 1059–1071
Wallace WA, Howie SE (1999) Immunoreactive interleukin 4 and interferon-gamma expression by type II alveolar epithelial cells in interstitial lung disease. J Pathol 187: 475–480
Wanders A, Wells AF, Larsson E, Tufveson G, Olsson T, Ljungdahl A, Klareskog L (1992) Expression of an interferon-gamma-like substance in normal and transplanted rat heart tissue. J Heart Lung Transpl 11: 142–146
Weber-Nordt RM, Mertelsmann R, Finke J (1998) The JAK–STAT pathway: signal transduction involved in proliferation, differentiation and transformation. Leuk Lymphoma 28: 459–467
Wei YP, Kita M, Shinmura K, Yan XQ, Fukuyama R, Fushiki S, Imanishi J (2000) Expression of IFN-gamma in cerebrovascular endothelial cells from aged mice. J Interferon Cytokine Res 20: 403–409
Acknowledgements
The present work was supported by grants from the Chinese Army Medical Research Council (02ma04).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Li, Q., Zhang, Xq., Nie, L. et al. Expression of interferon-γ in human adrenal gland and kidney tumours. Br J Cancer 97, 420–425 (2007). https://doi.org/10.1038/sj.bjc.6603870
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6603870