Abstract
Osteopontin is a multifunctional 34 kDa extracellular matrix protein with a cell-binding domain. It is involved cell adhesion and cell migration and is therefore considered to influence tumorigenesis and/or metastasis. The purpose of the present study was to evaluate the clinical significance of Osteopontin expression in oesophageal squamous cell carcinoma (ESCC). In the present study, we immunohistochemically investigated the relationship between Osteopontin expression and clinicopathological factors including prognosis in surgical specimens of primary tumours in 175 patients with ESCC. Osteopontin was expressed in 48% of 175 patients. Osteopontin expression was significantly correlated with lymph node metastasis, lymphatic invasion, and stage (P=0.0015, 0.037 and 0.033, respectively). Tumours with expressing Osteopontin exhibited more lymph node metastasis, lymphatic invasion and advanced stage than the tumour with negative Osteopontin expression. Five-year survival rate was better in patients with negative Osteopontin expression than in those with positive Osteopontin expression (P=0.035). However, multivariate analysis revealed that Osteopontin expression was not an independent prognostic factor. As our findings suggest that Osteopontin may play an important role in progress of ESCC, the evaluation of Osteopontin expression is useful for predicting the malignant properties of ESCC.
Similar content being viewed by others
Main
Osteopontin is a 34 kDa extracellular matrix protein with a cell-binding domain (Sodek et al, 2000) and was originally identified as a major component of the noncollagenous organic bone matrix. It secretes adhesive glycoprotein and seems to be involved in osteoblast differentiation, bone formation and remodelling of mineralised tissue (Reinholt et al, 1990; Giachelli and Steitz, 2000). Other molecules which share this domain include fibronectin, vitronectin and a variety of other extracellular proteins that bind members of the integrin family of cell surface receptors (Varner and Cheresh, 1996). The expression of Osteopontin has subsequently been demonstrated in a wide range of normal human tissue and body fluid such as osteoblasts, arterial smooth muscle cells, leukocytes, activated macrophages and T cells (Coppola et al, 2004), and epithelia of the gastro-intestinal tract (Brown et al, 1992; Brown et al, 1994). It is a multifunctional protein involved in cell adhesion and cell migration, and has been shown to play important roles in tumorigenesis, tumour invasion, metastasis and prognosis among patients with breast (Tuck et al, 1998), lung (Zhang et al, 2001; Donati et al, 2005), prostate (Thalmann et al, 1999), gastric (Ue et al, 1998) and colon cancer (Agrawal et al, 2002).
Oesophageal squamous cell carcinoma (ESCC) is one of the most aggressive carcinomas in the gastrointestinal tracts. Studies of various biological factors affecting the malignant potential of ESCC have been performed. However, the expression of Osteopontin in ESCC has not been evaluated. The aims of this retrospective study were to examine the expression of Osteopontin in surgical specimens of ESCC and to evaluate whether this is useful in predicting outcome.
Materials and methods
Patients and specimens
Subjects were 175 patients with ESCC (160 men and 15 women) who underwent oesophagectomy with lymph node dissection between 1987 and 1998 at Kagoshima University Hospital, Japan. The median age of the patients was 64 years (range 36–83 years). None of these patients underwent endoscopic mucosal resection, palliative resection, preoperative chemotherapy and/or radiotherapy, and none of them had synchronous or metachronous cancer in other organs. Specimens of cancer tissues and noncancerous adjacent tissue were collected from patients after informed consent had been obtained in accordance with the institutional guidelines of our hospital. Using the tumour node metastasis classification of the International Union Against Cancer (Sobin and Fleming, 1997), all of the M1 tumours exhibited distant lymph node metastases. Clinicopathologic data of patients in this study were shown in Table 1. All patients were followed-up after discharge, with X-ray examination and tumour marker assays (squamous cell carcinoma antigen and carcinoembryonic antigen) every 1–3 months, computed tomography every 3–6 months, and ultrasonography every 6 months. Bronchoscopy and endoscopy were performed when necessary. Postoperative follow-up data were obtained from all patients, with a median follow-up period of 28 months (range, 1–175 months).
Immunohistochemical staining and evaluation
Specimens were cut into 3-μm-thick sections, which were mounted on glass slides. Immunohistochemical staining was carried out using the avidin–biotin–peroxidase complex method (Vectastatin Elite ABC Kit; Vector, Burlingame, CA, USA), following the manufacturer's instructions. Briefly, the immunostaining was performed manually at room temperature. Sections were deparaffinised in xylene and dehydrated in ethanol, endogenous peroxidase activity was blocked by incubating sections for 10 min in 3% hydrogen peroxide in methanol. Sections then were then heated using an autoclave in a citrate buffer (0.01 mol l−1, pH 6.5) at 121°C for 15 min to reveal the antigen. After cooling, sections were preincubated in 1% borine serum albmin (BSA) for 20 min. Next, sections were incubated with antiosteopontin monoclonal antibody (1 : 50, Osteopontin, Novocastra Laboratories Ltd, Newcastle, UK) for 60 min. After rinsing with phosphate-buffered saline (PBS) for 15 min, sections were incubated with secondary antibody for 20 min and washed again with PBS for 10 min. Sections were incubated with avidin–biotin complex for 30 min and washed again, and reactions were visualized using diaminobenzidine tetrahydrochloride for 2 min. All samples were lightly counterstained with haematoxylin for 1 min. The negative controls consisted of sections treated with PBS instead of primary antibody. A section of normal tissue of gallbladder was used as positive control. Evaluation of immunohistochemistry was performed independently by two investigators (KY and SN). Positive Osteopontin expression was defined as detectable immunoreaction in perinuclear and other cytoplasmic regions of >10% of the cancer cells. To evaluate expression of Osteopontin, 10 fields (within the tumour and at the invasive front) were selected, and expression in 1000 tumour cells (100 cells/field) was evaluated using high-power (× 200) microscopy.
Statistical analysis
Statistical analysis of group differences was performed using the χ2 test. The Kaplan–Meier method was used for survival analysis, and differences in survival were estimated using the log rank test. Prognostic factors were examined by univariate and multivariate analyses (Cox proportional hazards regression model). P<0.05 was considered to indicate statistical significance. All statistical analyses were performed using the StatView™ for Windows software (Version 5.0, Abacus Concepts, Berkeley, CA, USA).
Results
Expression of Osteopontin in oesophageal squamous cell carcinoma
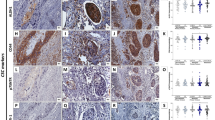
Osteopontin was expressed in the cytoplasm of ESCC cells in 48% (84 of 175) of all patients (Figure 1).
Relationship between Osteopontin and clinicopathologic variables
Osteopontin expression was significantly associated with the following clinicopathologic parameters: lymph node metastasis, stage and lymphatic invasion (Table 1). Patients with positive Osteopontin expression had more lymph node metastasis and greater lymphatic invasion than those with negative Osteopontin expression (P=0.0015 and 0.037, respectively).
Relationship between Osteopontin expression and prognosis
The 5-year survival rate was significantly lower in patients with positive Osteopontin expression than in those with negative Osteopontin expression (P=0.035; Figure 2).
Univariate and multivariate analyses of survival
Tables 2 and 3 show univariate and multivariate analyses of factors related to patient prognosis. Univariate analysis showed that the following factors were significantly related to postoperative survival: depth of invasion and lymph node metastasis, stage, lymphatic invasion, venous invasion and Osteopontin expression (P<0.05). Multivariate regression analysis indicated that depth of invasion, lymph node metastasis and venous invasion were independent prognostic factors, but that lymphatic invasion and Osteopontin expression were not independent prognostic factors.
Discussion
In this study, we immunohistochemically investigated the relationship between Osteopontin expression and clinicopathological factors, including prognosis, in ESCC. The expression of Osteopontin was observed in 48% of tumours. This was consistent with previous immunohistochemical studies on other carcinomas in which Osteopontin expression was detected in 33.3–70.0% (Ue et al, 1998; Zhang et al, 2001; Coppola et al, 2004).
In the present study, Osteopontin expression was significantly associated with lymph node metastasis and lymphatic invasion. In non-small cell lung cancer, the expression of Osteopontin in surgical specimens is significantly correlated with tumour size, lymph node metastasis and stage (Donati et al, 2005). In the ESCC, plasma Osteopontin was associated with lymph node metastasis (Shimada et al, 2005). In gastric cancer, the expression of Osteopontin in poorly differentiated tumours is also associated with lymphogenous metastasis (Ue et al, 1998). Taking these results together, it appears that the expression of Osteopontin in some human malignant tumours might be more associated with metastasis than with tumorigenesis.
The molecular mechanisms that define the role of Osteopontin in tumour metastasis have not been completely elucidated, although several mechanisms have been suggested. Previous studies showed that Osteopontin can support attachment for a variety of cell types and promote migration of tumour cells (Oates et al, 1997; Tuck et al, 2000). Osteopontin contains the cell attachment amino-acid sequence RGD (arginine–glycine–aspartic acid), which bind to the alpha vs beta 3 integrin heterodimer, play a role in cell adhesion (Xuan et al, 1995). In addition, Osteopontin bind several different cellular receptors, potentially allowing it to stimulate various signalling pathways and influence cellular events that may ultimately promote tumorgenesis, adhesion, migration and metastasis (Hu et al, 1995; Liaw et al, 1995; Weber et al, 1996; Al-Shami et al, 2005). The down-stream Osteopontin signals which interrupt the cell cycle, prevent apoptosis and promote cell survival are integral to tumour progression (Evan and Vousden, 2001). There may be especially signals that corresponded with lymph node metastasis in those signals that Osteopontin stimulate. In the present study, close relationship was found between Osteopontin expression and lymph node metastasis.
Concerning the survival analysis, sex, tumour depth, lymph node metastasis, stage, lymphatic invasion, venous invasion and Osteopontin expression were prognostic factors on univariate analysis. However, multivariate analysis revealed that only tumour depth, nodal metastasis and venous invasion were independent prognostic factors. It has previously been demonstrated that plasma Osteopontin in ESCC is associated with poor survival (Shimada et al, 2005). Expression of Osteopontin is also significantly associated with poor survival in stage I non-small cell lung cancer (Donati et al, 2005). These findings indicate that the Osteopontin overexpression in some tumours is correlated with poor prognosis, predominantly via lymph node metastasis. Although Osteopontin expression in ESCC was not found to be an independent prognostic factor in the present study, it might play an important role in promoting progression and migration to the lymphatic system. Thus, evaluation of Osteopontin expression using biopsy specimens before surgical therapy may be a new standard for appropriate lymph node dissection of ESCC.
In conclusion, we detected Osteopontin protein in ESCC and found this to be associated with lymph node metastasis, stage, lymphatic invasion, and prognosis. Osteopontin is therefore useful for predicting malignant properties. These findings suggested a possible role for Osteopontin expression level as a new diagnostic and prognostic biomarker for ESCC. Furthermore, understanding the biological function of Osteopontin expression in ESCC may help to determine its role in physiology of ESCC.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Agrawal D, Chen T, Irby R, Quackenbush J, Chambers AF, Szabo M, Cantor A, Coppola D, Yeatman TJ (2002) Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. J Natl Cancer Inst 94: 513–521
Al-Shami R, Sorensen ES, Ek-Rylander B, Andersson G, Carson DD, Farach-Carson MC (2005) Phosphorylated osteopontin promotes migration of human choriocarcinoma cells via a p70 S6 kinase-dependent pathway. J Cell Biochem 94: 1218–1233
Brown LF, Berse B, Van de Water L, Papadopoulos-Sergiou A, Perruzzi CA, Manseau EJ, Dvorak HF, Senger DR (1992) Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell 3: 1169–1180
Brown LF, Papadopoulos-Sergiou A, Berse B, Manseau EJ, Tognazzi K, Perruzzi CA, Dvorak HF, Senger DR (1994) Osteopontin expression and distribution in human carcinomas. Am J Pathol 145: 610–623
Coppola D, Szabo M, Boulware D, Muraca P, Alsarraj M, Chambers AF, Yeatman TJ (2004) Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res 10: 184–190
Donati V, Boldrini L, Dell'Omodarme M, Prati MC, Faviana P, Camacci T, Lucchi M, Mussi A, Santoro M, Basolo F, Fontanini G (2005) Osteopontin expression and prognostic significance in non-small cell lung cancer. Clin Cancer Res 11: 6459–6465
Evan GI, Vousden KH (2001) Proliferation, cell cycle and apoptosis in cancer. Nature 411: 342–348
Giachelli CM, Steitz S (2000) Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol 19: 615–622
Hu DD, Lin EC, Kovach NL, Hoyer JR, Smith JW (1995) A biochemical characterization of the binding of osteopontin to integrins alpha v beta 1 and alpha v beta 5. J Biol Chem 270: 26232–26238
Liaw L, Skinner MP, Raines EW, Ross R, Cheresh DA, Schwartz SM, Giachelli CM (1995) The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J Clin Invest 95: 713–724
Oates AJ, Barraclough R, Rudland PS (1997) The role of osteopontin in tumorigenesis and metastasis. Invasion Metastasis 17: 1–15
Reinholt FP, Hultenby K, Oldberg A, Heinegard D (1990) Osteopontin – a possible anchor of osteoclasts to bone. Proc Natl Acad Sci USA 87: 4473–4475
Shimada Y, Watanabe G, Kawamura J, Soma T, Okabe M, Ito T, Inoue H, Kondo M, Mori Y, Tanaka E, Imamura M (2005) Clinical significance of osteopontin in esophageal squamous cell carcinoma: comparison with common tumor markers. Oncology 68: 285–292
Sobin LH, Fleming ID (1997) TNM Classification of Malignant Tumors, fifth edition (1997) Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 80: 1803–1804
Sodek J, Ganss B, McKee MD (2000) Osteopontin. Crit Rev Oral Biol Med 11: 279–303
Thalmann GN, Sikes RA, Devoll RE, Kiefer JA, Markwalder R, Klima I, Farach-Carson CM, Studer UE, Chung LW (1999) Osteopontin: possible role in prostate cancer progression. Clin Cancer Res 5: 2271–2277
Tuck AB, Elliott BE, Hota C, Tremblay E, Chambers AF (2000) Osteopontin-induced, integrin-dependent migration of human mammary epithelial cells involves activation of the hepatocyte growth factor receptor (Met). J Cell Biochem 78: 465–475
Tuck AB, O'Malley FP, Singhal H, Harris JF, Tonkin KS, Kerkvliet N, Saad Z, Doig GS, Chambers AF (1998) Osteopontin expression in a group of lymph node negative breast cancer patients. Int J Cancer 79: 502–508
Ue T, Yokozaki H, Kitadai Y, Yamamoto S, Yasui W, Ishikawa T, Tahara E (1998) Co-expression of osteopontin and CD44v9 in gastric cancer. Int J Cancer 79: 127–132
Varner JA, Cheresh DA (1996) Integrins and cancer. Curr Opin Cell Biol 8: 724–730
Weber GF, Ashkar S, Glimcher MJ, Cantor H (1996) Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science 271: 509–512
Xuan JW, Hota C, Shigeyama Y, D'Errico JA, Somerman MJ, Chambers AF (1995) Site-directed mutagenesis of the arginine-glycine-aspartic acid sequence in osteopontin destroys cell adhesion and migration functions. J Cell Biochem 57: 680–690
Zhang J, Takahashi K, Takahashi F, Shimizu K, Ohshita F, Kameda Y, Maeda K, Nishio K, Fukuchi Y (2001) Differential osteopontin expression in lung cancer. Cancer Lett 171: 215–222
Acknowledgements
This study was supported in part by grants-in-aid for scientific research from the Ministry of Education, Science, Sports and Culture, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Kita, Y., Natsugoe, S., Okumura, H. et al. Expression of Osteopontin in oesophageal squamous cell carcinoma. Br J Cancer 95, 634–638 (2006). https://doi.org/10.1038/sj.bjc.6603296
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6603296
Keywords
This article is cited by
-
Osteopontin is a prognostic circulating biomarker in patients with neuroendocrine neoplasms
Journal of Cancer Research and Clinical Oncology (2023)
-
Serum anti-SPP1 autoantibody as a potential novel biomarker in detection of esophageal squamous cell carcinoma
BMC Cancer (2022)
-
Identification of four genes and biological characteristics of esophageal squamous cell carcinoma by integrated bioinformatics analysis
Cancer Cell International (2021)
-
Prognostic factors in esophageal squamous cell carcinoma patients treated with neoadjuvant chemoradiation therapy
International Journal of Clinical Oncology (2013)
-
Osteopontin Expression in Squamous Cell Cancer of the Esophagus
World Journal of Surgery (2008)