Abstract
The hysterectomy-corrected age-adjusted incidence rate of endometrial cancer was 29%, and for cervical cancer 11% higher than the uncorrected rate. Correction factors for such cancer sites are recommended for regular use. The levelling-off of the incidence of endometrial cancer appears to be an artefact caused by the increasing prevalence of hysterectomy.
Similar content being viewed by others
Main
Hysterectomy is one of the commonest surgical procedures for women with substantial regional and international variations in frequency (Carlson et al, 1993; Luoto et al, 1994; Settnes and Jorgensen, 1999). The proportion of women who have undergone a hysterectomy increases with age up to 55–59 years (van Keep et al, 1983; Luoto et al, 1994). The highest rates are in the United States, where approximately a third of women aged 45 years and over have undergone this operation. In Finland and in the United Kingdom, the corresponding fraction is at ages 45–64 years is approximately one-fifth (Walker and Jick, 1979; Vessey et al, 1992; Luoto et al, 1994; Clarke et al, 1995). In spite of reliable surveillance of hysterectomy rates, cancer rates have not been corrected for hysterectomy (Luoto et al, 1994).
We have used information on hysterectomy to derive uteri-at-risk and cervices-at-risk populations to correct gynaecological cancer rates in Finland.
Materials and methods
To estimate the incidence of hysterectomy during the period 1950–1986, a table with 1-year calendar years as rows and 1-year age groups as columns, each cell including the number of cases and the population at risk, was formulated, from which birth cohort-specific rates could be derived.
The Finnish Hospital Discharge Register (FHDR) includes information on surgical procedures since 1987, but the codes were unreliable until 1990. Coding of hysterectomies was based on Nordic Version of ICD-9 coding of surgical procedures during the period 1987–1996, from 1997 on ICD-10 classification. For the present study, incidence rates for 5-year age groups and each year on 1991–1999 were calculated from FHDR.
The fit of the model-based prevalences was tested against prevalence rates of hysterectomy based on self-reports from three population-based health surveys in 1981, 1989 and 1997, with nearly identical questions on hysterectomy was nearly identical, including structural options on hysterectomy alone or with oophorectomy. First, the sample of Mini-Finland Health Study in 1981 included 8000 persons 30–99 years of age, half of which were women. Almost 90% of them underwent both health interview and clinical examination (Aromaa et al, 1989). In 1989, prevalence of hysterectomy was obtained from a mail questionnaire survey among 45- to 64-year-old Finnish women (N=2000) randomly sampled from the Population Register. The response rate was 86% (Luoto et al, 1994). Third point prevalence of hysterectomy was based on the FINRISK mail questionnaire survey in 1997, covering a random sample of population in five regions: two in eastern, one in southwestern and one in Northern Finland together with one in the Helsinki capital area (Vartiainen et al, 1998). Response rate was 75% among the 3763 of female respondents aged 25–64 years.
Corrected estimates of endometrial, cervical and ovarian cancer incidence were produced by multiplying the cancer incidence rates in 5-year age groups by a correction factor (the percentage of hysterectomised women). The Poisson's model including interaction between cohort and period was extrapolated for the years 2000–2010 to obtain future rates.
Results
Incidence and cumulative incidence of hysterectomies
From 1991 to 1999, the annual number of hysterectomies (11 956 in 1999) increased by 16% (Table 1). The incidence of partial hysterectomies (hysterectomies with cervix intact) increased from 2500 in 1991 to 3017 per million women in 1999. The age-specific incidence of hysterectomy increased until the 1990s, but changed in the 1990s to a decrease among the fertile aged women (age <50 years). In the birth cohort-specific hysterectomy rates, an increase up to the birth cohort born 1943–1947 was observed, after which the incidence of hysterectomy started to decrease (Figure 1). The first peak age at hysterectomy was observed around 47 years and the second in the age group 70–80 years.
Cumulative incidence rates (later referred to as ‘prevalence’) of hysterectomy in the age group 45–49 years have decreased since 1989 (Figure 2). The prevalence of hysterectomy continues to increase in age group 45–64 years of age, and even more steeply among older women of age 60–64 years of age. However, even in the oldest group this decreases after the year 2007. The estimated prevalence was mostly parallel to earlier published rates except in 1989 for the age group 60–64 years (Table 2).
Age-specific prevalence rates of hysterectomy in 1950–2010 in age groups 45–49 years (black line) and 60–64 years (grey line), based on estimated incidence rates. The vertical bars indicate the respective point prevalence rates of the three population-based surveys (Aromaa et al, 1989, Luoto et al, 1994, Vartiainen et al, 1998) together with their 95% confidence intervals. Dotted line shows the time trend of age-adjusted prevalence of hysterectomy in age range of 45–64 years.
Cancer incidence
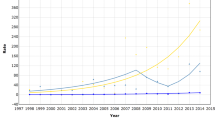
The age-adjusted incidence of endometrial cancer in 1999 was 146/106, but 188 per million after applying uteri-at-risk corrections (Table 1). Uncorrected endometrial cancer rates show a levelling-off in the 1980s, which is less apparent in corrected rates (Figure 3).
In the age group 50–59 years, the difference between uncorrected and corrected rate of endometrial cancer is large and up to 32% (Table 3). The impact of correction is smaller in cervical cancer, but even here the corrected rate of cervical cancer differs by up to 17.8%. The corrected age-adjusted trend in the incidence of cervical cancer was almost unchanged as compared to the uncorrected trend (Figure 4).
Discussion
When the corrected population at risk was used rather than the entire population, the incidence of endometrial cancer was 29%, cervical cancer 11% and ovarian cancer 8% higher. Uncorrected endometrial cancer rates showed a levelling-off in the 1980s not seen in corrected rates, explained by the increasing prevalence of hysterectomy a change not recognised previously. Such correction has not been systematically used in any country, although the first recommendations for correction were published 24 years ago (Lyon and Gardner, 1977) and the topic is still discussed (Merrill et al, 2001).
Our results were in keeping with the first US studies in the 1970s, showing a 20–45% increase in endometrial cancer incidence rate depending on the age group and calendar year (Lyon and Gardner, 1977; Merrill and Feuer, 1996) applied a risk-adjusted incidence method for cervical cancer and found that in the period 1990–1992 the age-adjusted incidence rate increased by 15%. The greatest increase, by 58%, was seen among women aged 65–69 years. Correction for the scale of hysterectomies in Canada in 1964–1966 to 1974–1966 converted an apparent fall in mortality from cancer of the endometrium to stability (Miller et al, 1981).
Our estimates of hysterectomy prevalence were based on reliable data on hysterectomy incidences over a 12-year period, obtained from a population-based hospital discharge register. The incidence trends were also extrapolated backwards to obtain estimates over the preceding life of the women. The validity of these extrapolations was confirmed by comparing the cumulative incidence rates with age-specific prevalence rates available from three cross-sectional population surveys.
The small surveys samples (especially that in 1989) made the point prevalences of hysterectomy relatively imprecise. However, our estimates fall within their intervals except for that in 1989 for the age group 60–64 years, with estimated prevalence markedly lower than that found. On the other hand, our estimated cumulative incidence rates were consistent with the two other survey results for this age group.
Coverage of FHDR is at least 90% compared to hospital records (Keskimäki and Aro, 1991). Generally, benefits of register information compared with cross-sectional surveys are clear, although cross-sectional hysterectomy results combined with mortality data analysed using a life-table method can produce similar results (Merrill, 2001, Merrill et al, 2001).
The incidence of hysterectomy is higher in Finland than in other Nordic countries (Luoto et al, 1994, Vuorma et al, 1998) is possibly related both to its health-care system with private gynaecologists and the increasing use of hormonal replacement therapy. Every third woman in Finland is currently using hormonal replacement therapy (Topo et al, 1999), which is known to enhance uterine fibroid proliferation, the most frequent indication for hysterectomy.
Indications for, and time trends of, hysterectomy vary in different countries, although differences are not large. Correction factors which therefore need to be country specific and stratified by age and time have been developed from 1998 onwards (Giersiepen et al, 2003). In those European countries where neither hospital-based data nor reproductive health surveys are available, a special survey is needed to obtain correction factors. Historical data on hysterectomy rates, required for correcting incidence trends, may be available only from register-based information sources as used in this study. In Finland, the correction has a material effect only on the incidence of endometrial cancer: the levelling-off in the 1980s of the incidence seems not to be real but an artefact caused by the increasing prevalence of hysterectomies.
A recent comparison of hospital discharge data and cross-sectional survey for correcting uterine cancer incidence (Merrill et al, 2001) concluded that both methods may provide correct estimated of burden of gynaecological cancer. The results of the present study confirm this view.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Aromaa A, Heliövaara M, Impivaara O, Knekt P, Maatela J, Joukamaa M, Klaukka T, Lehtinen V, Melkas T, Mälkiä E, Nyman K, Paunio I, Reunanen A, Sievers K, Kalimo E, Kallio V (1989) Terveys, to imintakyky ja hoidontarve Suomessa Mini-Suomi – terveystutkimuksen perustulokset. Kansaneläkelaitoksen julkaisuja, AL:32, Helsinki
Carlson KJ, Nichols DH, Schiff I (1993) Indications for hysterectomy. N Engl J Med 328: 856–860
Clarke A, Black N, Rowe P, Mott S, Howle K (1995) Indications for and outcome of total abdominal hysterectomy for benign disease: a prospective cohort study. Br J Obstet Gynecol 102: 611–620
Giersiepen K, Luoto R, Andrea E (ed) 2003 Hysterectomy Prevalence and Its Effect on Uterine Cancer Incidence Registration. Berlin: VWF
Keskimäki I, Aro S (1991) Accuracy of data on diagnoses, procedures and accidents in the Finnish hospital discharge register. Int J Health Sci 2: 15–21
Luoto R, Hemminki E, Topo P, Uutela A, Kangas I (1992) Hysterectomy among Finnish women – prevalence and women's own opinions. Scand J Soc Med 20: 209–212
Luoto R, Kaprio J, Keskimäki I, Pohjanlahti J-P, Rutanen E-M (1994) Hysterectomy in Finland 1987–1989 – incidence, indications and surgical approaches. Int J Epidemiol 23: 348–358
Lyon JL, Gardner JW (1977) The rising frequency of hysterectomy: its effect on uterine cancer rates. Am J Epidemiol 105: 439–443
Merrill RM (2001) Prevalence corrected hysterectomy rates and probabilities in Utah. Ann Epidemiol 11: 127–135
Merrill RM, Feuer EJ (1996) Risk-adjusted cancer-incidence rates (United States). Cancer Causes Control 7: 544–552
Merrill RM, Lyon JL, Wiggins C (2001) Comparison of two methods on cross-sectional data for correcting corpus uterine cancer incidence and probabilities. BMC Cancer 1: 13 (http://www.biomedcentral.com/1471-2407/1/13)
Miller AB, Visentin T, Howe GR (1981) The effect of hysterectomies and screening on mortality from cancer of the uterus in Canada. Int J Cancer 27: 651–657
Settnes A, Jorgensen T (1999) Hysterectomy in a Danish cohort. Prevalence, incidence and socio-demographic characteristics. Acta Obstet Gynecol Scand 75: 274–280
Topo P, Luoto R, Hemminki E, Uutela A (1999) Declining socioeconomic differences of in the use of menopausal and postmenopausal hormone therapy in Finland. Maturitas 32: 141–145
van Keep PA, Wildemeersch D, Lehert P (1983) Hysterectomy in six European countries. Maturitas 5: 69–75
Vartiainen E, Jousilahti P, Juolevi A et al. (1998) [Study on chronic disease risk factors, health behaviour, symptoms and use of health services. Design and basic results]. (in Finnish) Publications of the National Public Health Institute B 1998 http://www.ktl.fi/eteo/ktey/finriski97perusraportti.html
Vessey MP, Villard-Mackintosh L, McPherson K, Oculter A, Yeates D (1992) The epidemiology of hysterectomy: findings in a large cohort study. Br J Obstet Gynecol 99: 402–407
Vuorma S, Teperi J, Hurskainen R, Keskimäki I, Kujansuu E (1998) Hysterectomy trends in Finland in 1987–1995 – a register based analysis. Acta Obstet Gynecol Scand 77: 770–776
Walker AM, Jick H (1979) Temporal and regional variation in hysterectomy rates in the United States, 1970–1975. Am J Epidemiol 110: 41–46
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc).
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Luoto, R., Raitanen, J., Pukkala, E. et al. Effect of hysterectomy on incidence trends of endometrial and cervical cancer in Finland 1953–2010. Br J Cancer 90, 1756–1759 (2004). https://doi.org/10.1038/sj.bjc.6601763
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6601763
Keywords
This article is cited by
-
Social factors and age play a significant role in cervical cancer and advanced-stage disease among Danish women
BMC Cancer (2024)
-
Hysterectomy-corrected rates of endometrial cancer among women younger than age 50 in the United States
Cancer Causes & Control (2018)
-
Impact of hysterectomy and bilateral oophorectomy prevalence on rates of cervical, uterine, and ovarian cancer among American Indian and Alaska Native women, 1999–2004
Cancer Causes & Control (2011)
-
Hormone therapy and ovarian cancer: incidence and survival
Cancer Causes & Control (2008)