Abstract
Cell cycle progression in eukaryotic cells is regulated by a family of cyclin-dependent kinases (CDKs). Cyclin E is a regulatory subunit of CDK2 and drives cells from G1 to S phase. Increased expression of cyclin E is a frequent event in human malignancies and has been associated with poor prognosis in various cancers. In this study, we evaluated the effects of cyclin E-overexpression on the sensitivity of rat fibroblasts to anticancer drugs. Cyclin E-overexpressing cells were less sensitive to doxorubicin-induced inhibition of cell growth but not to other antineoplastic drugs, such as paclitaxel, vincristine, etoposide and methotrexate. Cyclin E-overexpressing fibroblasts also displayed a reduction in ROS levels and a significantly lower increase following doxorubicin treatment compared with vector control cells. The expression of manganese superoxide dismutase (MnSOD) and its activity were increased (about 1.3-fold) in cyclin E-overexpressing derivatives compared with control cells. These results suggest that cyclin E overexpression might reduce tumour cells sensitivity to doxorubicin by affecting the expression of MnSOD and that determination of cyclin E expression levels might help to select patients to be treated with an anthracycline-based antineoplastic therapy.
Similar content being viewed by others
Main
In mammalian cells, progression through the different phases of the cell cycle is regulated by the activity of a series of cyclin-dependent kinases (CDKs) that are activated by cyclins, their regulatory subunits. Cyclin E is a G1 cyclin expressed near the G1 to S transition and drives entry into the S phase by binding to and activating CDK2. Accumulation of cyclin E in late G1 is achieved by periodic transcription coupled with regulated ubiquitin-mediated proteolysis of the protein (Clurman et al, 1996). The proper timing and amplitude of cyclin E expression are important since their alterations cause deregulation of cell growth (Ohtsubo et al, 1995; Sgambato et al, 1997). Moreover, transgenic mice overexpressing cyclin E develop breast cancers (Bortner and Rosenberg, 1997) and increased levels of cyclin E have been reported in a variety of human malignancies, (Donnellan and Chetty, 1999; Sandhu and Slingerland, 2000).
The treatment of cancer patients with chemotherapy is often limited by the occurrence of cancer cells resistance. Recent data suggest a possible link between cell cycle alterations and the emergence of resistance to specific anticancer agents, although the molecular mechanisms underlying such correlation still need to be elucidated (Hochhauser et al, 1996; Eymin et al, 1999).
Doxorubicin is one of the most effective agents in the treatment of breast cancer, belonging to the anthracycline family of antitumour antibiotics. This drug inhibits topoisomerase II and promotes the formation of DNA double-strand breaks; moreover, it induces, as a byproduct of its enzymatic activation, the formation of reactive oxygen species (ROS), which are believed to contribute significantly to the cytotoxic activity of the compound (Doroshow, 1983). Oxygen-derived reactive species, such as superoxide anion radical (O2•−) and hydrogen peroxide (H2O2), are important mediators of several types of cell damage, their deleterious action being physiologically counteracted by antioxidant cell defences, including vitamins, glutathione and antioxidant enzymes (Galeotti et al, 1990). Primary antioxidant enzymes include superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX). Sodium dismutase converts O2•− into H2O2, which is then converted into water by CAT and GPX. There are two major forms of SOD in eukaryotic cells, a copper and zinc, containing enzyme (CuZnSOD), localised in the nucleus and cytoplasm, and a manganese-containing enzyme (MnSOD) present in the mitochondria (Galeotti et al, 1990). We and others previously reported that increased expression of MnSOD is associated with increased resistance to different kinds of oxidative stress. This is in particular true for cells exposed to redox-active pesticides (Paraquat) and, interestingly, to doxorubicin (Pani et al, 2000). These observations strongly suggest that oxidants have a major role in the cytotoxic effect of this compound, and, by extension, that antioxidant defences may contribute to cancer cell resistance to doxorubicin and other pro-oxidant drugs in vivo.
In the present study, we investigated the relations between the expression of cyclin E and cell response to anticancer drugs and found that increased cyclin E expression is associated with resistance to doxorubicin. We also found that this effect is associated with a reduced endogenous level of ROS and an increased expression of MnSOD. The implications of these findings are discussed.
Materials and methods
Cell culture
The Rat-1 diploid immortalised rat fibroblasts were grown and maintained in Eagle's minimum essential medium (EMEM) (Gibco, Merelbeke, Belgium) supplemented with 10% heat-inactivated FBS. Doubling times were calculated from the initial exponential phase of the growth curves. Briefly, cells were plated at a density of 1 × 104 cells per 35 mm diameter well, in triplicate, and the number of cells per well was determined every day using a cell counter.
Chemicals
Purified doxorubicin, etoposide (VP-16), methotrexate, vincristine, paclitaxel and Paraquat-dichloride were purchased from Sigma (St Louis, MO, USA). Stock solution (10 mg/ml) was prepared and storaged according with the instructions of the supplier.
Construction of retrovirus vectors and viral transduction
The construction of the cyclin E retroviral expression plasmid PMV12-cycE and the method used for retrovirus packaging and transduction have been previously described (Sgambato et al, 1996, 1997). Briefly, the full-length cyclin E cDNA was subcloned into the retroviral vector PMV12 in the sense orientation (Cacace et al, 1993). To prepare infectious retrovirus particles, the PMV12-cycE plasmid or the control vector PMV12pl was transfected into the Ψ2 ecotropic retrovirus packaging cell lines. The transfected cells were selected by growth in hygromycin and the cell-free media, containing defective recombinant viruses, were harvested, filtered and used for the infection. Following selection for cells resistant to hygromycin, several pools of thousands of resistant colonies were obtained and used for further analysis.
Cytotoxicity assay
Cells were plated in triplicate in 24-well plates at a density of 2 × 104 cells per well and allowed to adhere 24–36 h before drug treatment. They were then rinsed and grown in medium supplemented with increasing concentrations (from 0 to 10 mM) of each drug. After 48 h, the medium was removed and cultures were incubated with medium containing 1 mg ml−1 MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; Sigma) for 2 h at 37°C. The medium was then discarded and 500 μl acid-isopropanol (0.04 N HCL in isopropanol) was added to each well to stop the cleavage of the tetrazolium ring by dehydrogenase enzymes that convert MTT to an insoluble purple formazan in living cells. Plates were then kept in agitation at room temperature for about 15–20 min and the level of the coloured formazan derivative was determined on a multiscan reader at a wavelength of 540 nm (reference wavelength 630 nm). Clonogenic assay was performed by seeding 500 and 1000 cells per 10 cm dish in complete medium. After overnight incubation, when cells were attached, but had not yet divided, medium was removed and, after washing with PBS, doxorubicin was added for 24 or 48 h. Afterwards, medium was changed and cultures were refed with fresh medium every 3–4 days for about 2 weeks. The cells were then fixed and stained with Giemsa and the number of grossly visible colonies was counted.
Western blot analysis and MnSOD activity
Exponentially growing cultures of each cell lines were collected by cell scraping and cell pellets were added to 3–5 volumes of sonication buffer containing proteases and phosphatase inhibitors and sonicated at 4°C, as previously described (Sgambato et al, 1997; Pani et al, 2000). Homogenates were incubated in ice for 30 min and then centrifuged at 14 000 rpm for 15 min at 4°C. The supernatants were assayed for protein content and 50 μg of protein from each sample was separated by SDS–PAGE and transferred to immobilon-P membranes. Immunodetection was performed using the enhanced chemiluminescence kit for Western blotting detection (Amersham). Assays for cyclin E-associated histone H1 kinase activity were performed as previously described (Sgambato et al, 1997). The polyclonal antibody to cyclin E was obtained from Upstate Biotechnology (Lake Placid, NY, USA). Anti-MnSOD and anti-CuZnSOD antibodies were from Calbiochem (Merck Eurolab GmbH, Germany). Bands were analysed on the image analysis system Gel Doc 200 System (Biorad Laboratories S.r.l., Milan, Italy) and quantitated using the Quantity One Quantitaton Software (Biorad). Manganese superoxide dismutase activity was evaluated by ‘in gel’ SOD assay on 100 μg of total protein lysates, as previously described (Beauchamp and Fridovich, 1971; Pani et al, 2000).
Determination of DNA content by FACS analysis
Cells were plated in duplicate in 6-cm dishes at a density of 5 × 105 cells per dish and incubated 48 h. They were then trypsinised, collected and washed twice with PBS. Cell pellets were resuspended in 1 ml PBS and fixed in 5 ml of 70% ethanol. For the analysis, cells were collected by centrifugation and the pellets were resuspended in 0.2 mg ml−1 of propidium iodide in HBSS containing 0.6% Nonidet P-40 and RNase (50 μg ml−1). After incubation in the dark at room temperature for 30–60 min, the cell suspension was filtered and analysed for DNA content on a Coulter EPICS 753 flow cytometer. The per cent of cells in different phases of the cell cycle was determined using the Multycicle software version 2.53.
Measurement of ROS production
Intracellular ROS production was measured in cyclin E-overexpressing and in control cells using the oxygen radical-sensitive probe dichlorodihydrofluorescein diacetate (DCF) (Molecular Probes, Inc., Eugene, OR, USA) as described (Sgambato et al, 2001). Fluorescent units were measured in each well at 15-min interval for 45 min following incubation with DCF (10 μ M), using a Cytofluor™ 2300/2350 Fluorescence Measurement System (Millipore Corp., USA) with an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
Intracellular ROS concentration was also assessed by flow cytometry on cells loaded with DCF. The dye (10 μg ml−1) was added to cell culture 30 min before analysis. Cells were then trypsinised and green fluorescence was analysed using a Coulter Epics flow cytometer equipped with a 480 nm emission laser.
Statistical analysis
Mean values were compared using the Wilcoxon's test. Calculations were performed using the STATA 6.0 statistical software package (Stata Corporation, College Station, TX, USA) and the results were considered statistically significant when the P-value was ⩽0.05.
Results
Increased expression of cyclin E stimulates the growth of rat fibroblasts
To obtain cyclin E-overexpressing derivatives of Rat-1 fibroblasts, cells were transduced with a human cyclin E cDNA expressed from a retroviral promoter, as previously described. After selection, a pool of thousands of resistant colonies was obtained both from the cultures infected with the cyclin E cDNA construct (Rat-E) and the cultures infected with the empty vector (Rat-V, vector control cells), respectively. Pools of transfected cells were used for these studies, rather than single clones, to eliminate the possibility that our results were due to clonal heterogeneity commonly observed in cultured cells.
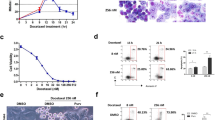
Expression of the exogenous cyclin E gene was verified by Western blot analysis with a specific antibody to cyclin E. The major endogenous cyclin E band was about 55 kDa in the rat fibroblasts. A strong band of about 50 kDa corresponding to the exogenous cyclin E was also detected in the overexpressing pool but not in the vector control cells (Figure 1). The size difference between the human and rodent forms was a convenient property for distinguishing the endogenous and exogenous cyclin E. To determine the effects of cyclin E overexpression on CDK activity, lysates from both pools were assayed for cyclin E-associated kinase activity, which resulted in significantly higher levels in Rat-E cells compared with vector control cells (Figure 1). No differences were observed in the doubling time of cyclin E-overexpressing cells compared with Rat-V control and parental cells (about 18 h, data not shown). However, as expected, the cyclin E-overexpressing derivatives of Rat-1 fibroblasts displayed a reduction in the percentage of cells in the G1 phase (about 36 vs 62%) and an increase in the percentage of cells in the S phase (about 47 vs 22%), when compared to the vector control cells (Table 1). As shown, no significant differences in cell cycle distribution were observed between Rat-V and Rat-1 parental cells. Based on the length of the exponential doubling time, we used the flow cytometry data to calculate the approximate length in hours of each of the phases of the cell cycle in the two cell lines. As shown in Table 1, the cyclin E-overexpressing cells displayed a shortening of the G1 phase associated with lengthening of the S phase of the cell cycle.
Levels of expression of cyclin E and cyclin E-associated kinase activity in Rat-V and Rat-E cells. Top panel, cell extracts from exponentially growing cultures of both cell lines were analysed for the expression of cyclin E using a specific anticyclin E antibody. The major endogenous cyclin E band was about 55 kDa in the rat fibroblasts. A strong band of about 50 kDa corresponding to the exogenous cyclin E was detected in the overexpressing pool. The size difference between the human and rodent forms was a convenient property for distinguishing the endogenous and exogenous cyclin E. Bottom panel, cell extracts from both cell lines were assayed for the cyclin E-associated histone H1 kinase activity.
Cyclin E overexpression is associated with an increased resistance to doxorubicin but not to other antineoplastic agents
To evaluate whether the increase in cyclin E expression affected sensitivity of cells to anticancer drugs, exponentially growing cultures of both vector control and cyclin E-overexpressing derivatives were exposed to increasing concentration of drugs (methotrexate, etoposide, paclitaxel, vincristine and doxorubicin) for 48 h and the concentration inhibiting cell growth by 50% (IC50) was determined. Cytotoxicity assays were carried out by use of the MTT test. A dose-dependent decrease in cell number was observed with all the five drugs. As shown in Figure 2A, cyclin E-overexpressing fibroblasts were more resistant to doxorubicin compared with vector control cells (the IC50 value was 1.9-fold higher) (P<0.0001). The effect was specific since no significant difference in growth inhibition was evident between Rat-V and Rat-E for methotrexate, etoposide, paclitaxel and vincristine even when drug treatment was prolonged up to 72 h (Table 2 and data not shown). The increased resistance of cyclin E-overexpressing derivatives was also evident after 72 h incubation with doxorubicin (IC50=122.1±9.7 and 193.3±20.1, respectively) (P<0.0001) (data not shown). Confirmatory results were obtained when Rat-E and Rat-V cells were incubated in media at different concentration of doxorubicin and cell growth was analysed up to 72 h by cell counting (data not shown). Chemosensitivity to doxorubicin was also assessed by clonogenic assay, which is a better predictor of in vivo sensitivity compared with MTT test. Different concentrations of drug were tested for 24 and 48 h treatment and inhibition in colony formation was always higher for control compared with cyclin E-overexpressing cells (data not shown). At a concentration of 25 nM doxorubicin, colony formation was reduced about 20 (19.3±4) and 11 (10.8±2)% after 24 h and 30 (29.5±5) and 13 (12.7±4)% after 48 h treatment for Rat-V and Rat-E cells, respectively, compared with untreated cells (Figure 2B). We analysed the effects of drug treatment on cell cycle distribution and found that treatment of cells with doxorubicin (IC50) for 24 h mainly caused an accumulation of cells in the G1 phase of the cell cycle in both the Rat-V (G0/G1=73.4; S=14.7; G2/M=11.9) and Rat-E cells (G0/G1=60.4; S=26.8; G2/M=12.8), compared with untreated cells. We also looked for apoptosis by FACS and TUNEL analysis in doxorubicin-treated cells, but were unable to detect apoptotic cells in both Rat-V and Rat-E cells up to 48 h treatment with the IC50. We cannot exclude that apoptosis might be apparent at later time points or with higher drug concentration (data not shown).
Increased resistance of cyclin E-overexpressing cells to doxorubicin-induced inhibition of cell growth. (A) Cells were exposed to the indicated concentration of doxorubicin and cell numbers were estimated by the MTT test after 48 h and plotted as a function of controls without drugs. (B) Chemosensitivity to doxorubicin was assessed by clonogenic assay. Cells were exposed to doxorubicin for 24 and 48 h and results were expressed as percentage of inhibition in colony formation in drug-treated compared with untreated cells. See Materials and Methods for details.
Increased expression of cyclin E is associated with a reduction in ROS
Doxorubicin belongs to anthracycline antibiotics that have been shown to induce cytotoxicity by generating ROS and whose activity is influenced by the redox state of cells (Doroshow, 1983; Sinha et al, 1987; Friesen et al, 1999).
To evaluate whether cyclin E overexpression was associated with changes in the redox state of rat fibroblasts, we analysed the oxidative state of both cyclin E-overexpressing and control cells using the fluorescent probe DCF, which is a sensitive fluorimetric probe of the production of oxidative stress in living cells (Zhu et al, 1994). We found that the basal level of endogenous ROS was significantly lower (about 16%) (P=0.001) in cyclin E-overexpressing cells compared with vector control and parental cells (Figure 3A). The reduced level of ROS was also confirmed using a fluorescence microscope (data not shown) and by flow cytometric (FACS) analysis of cells after incubation with DCF for 45 min (Figure 3B).
Reduced concentration of intracellular ROS in cyclin E-overexpressing cells. (A) dichlorodihydrofluorescein diacetate fluorescence was measured after 45 min incubation with DCF (10 μ M). The values represent the means±s.d. of four different experiments performed in triplicate and are shown as per cent of Rat-1 control cells. Similar values were obtained taking into account the readings after 15 and 30 min incubation with DCF (data not shown). *Significant at P=0.001 for Rat-E vs Rat-V. (B) Intracellular ROS concentration was assessed by flow cytometry on cells loaded with DCF. The dye (10 μg ml−1) was added to cell culture 30 min before analysis. Cells were then detached from the substrate and green fluorescence was analysed using a Coulter Epics flow cytometer equipped with a 480 nm emission laser. Numbers beside the histograms indicate mean cell fluorescence and are the averages of three independent experiments.
Reduced induction of free radical production by doxorubicin in cyclin E-overexpressing cells
Since generation of ROS has been suggested as a main mechanism of anthracycline cytotoxicity (Doroshow, 1983; Sinha et al, 1987), we asked whether doxorubicin-induced increase in ROS level was also reduced in cyclin E-overexpressing cells compared with vector control cells. Although both cell lines displayed a marked increase in ROS levels following doxorubicin treatment, it was about 1.9-fold for Rat-V and 1.7-fold for Rat-E cells with an increase of 107 and 67 fluorescence units, respectively. It is of interest, however, that the absolute values remained constantly lower in Rat-E compared to Rat-V cells after drug treatment (P=0.03 after 4 h by Wilcoxon test) (Table 3). This effect was not specific for doxorubicin. In fact, exposure to H2O2 (100 μ M for 15 min) also caused a smaller increase in ROS level in cyclin E-overexpressing cells compared to vector control cells (P=0.003) (Table 3).
Cyclin E overexpression is associated with an increased expression of MnSOD
Manganese superoxide dismutase is the principal scavenger for superoxide in mitochondria (Fridovich, 1998). Since increased expression of MnSOD has been associated with improved survival of cells to doxorubicin (Hirose et al, 1993), we aimed to verify whether changes in the expression level of this protein played a role in the increased resistance of cyclin E-overexpressing fibroblast to doxorubicin. The expression of MnSOD was evaluated by Western blot analysis in Rat-E and Rat-V cells. As shown in Figure 4A and B, cyclin E-overexpressing derivatives expressed an increased level (about 1.3-fold) of expression of MnSOD protein compared with Rat-V vector control cells. Accordingly, MnSOD activity was also significantly increased (about 40%) in Rat-E cells, compared with control cells, as assessed by gel SOD assay (Figure 4C). No differences were observed in the expression of CuZnSOD (Figure 4A).
Increased expression and activity of MnSOD in cyclin E-overexpressing cells. (A) Cell extracts from exponentially growing cultures of both the cell lines were analysed for the expression of MnSOD and CuZnSOD by Western blot analysis. (B) Densitometric analysis of MnSOD bands. The values shown are the ratios of MnSOD to β-actin obtained in two independent experiments. (C) Manganese superoxide dismutase activity was assessed by gel SOD assay, as described by Beauchamp and Fridovich (1971).
Discussion
In this study, we demonstrated that cyclin E overexpression, which is a frequent event in a variety of human malignancies, is associated with an increased resistance to doxorubicin-induced cell death. Cyclin E is a major regulator of G1 to S transition in eukaryotic cells (Koff et al, 1991; Ohtsubo et al, 1995) and its increased expression has been reported in a variety of human malignancies (Donnellan and Chetty, 1999; Sandhu and Slingerland, 2000). In this study, we analysed the sensitivity to doxorubicin and to other anticancer drugs of derivatives of Rat-1 fibroblasts that stably overexpress cyclin E and demonstrated that changes in the expression levels of cyclin E can affect cell sensitivity to doxorubicin.
Doxorubicin is a chemotherapeutic agent widely used in the treatment of human cancers (DeVita et al, 2001). It belongs to the anthracycline family of anticancer antibiotics that can inhibit topoisomerase II action resulting in double-strand DNA breaks (Kasahara et al, 1992). However, the generation of ROS has been suggested as the major mechanism of anthracycline cytotoxicity. The quinone, which is functionally common to the members of this family, may undergo a one-electron reduction to the corresponding semiquinone free radical by flavin-centred reductases including NADH dehydrogenase (Doroshow, 1983). In the presence of oxygen, this free radical will trigger the generation of highly ROS that can induce lipid peroxidation and DNA damage. The enzymes catalase, SOD and the glutathione system play a key role in the cellular defence against ROS damage by detoxifying some of these reactive molecules before they react with vulnerable cellular targets (Fridovich, 1998). ROS generation as a result of doxorubicin treatment and prevention of its cytotoxicity by oxygen radical scavengers was directly demonstrated in MCF-7 human breast cancer cells (Doroshow, 1986; Bustamante et al, 1990).
The present study demonstrates that increased expression of cyclin E in rat fibroblasts is associated with an increased resistance to doxorubicin, but not to other antineoplastic agents, such as methotrexate, etoposide, vincristine and cysplatin. We also demonstrated that this effect likely relates to a reduced basal level of endogenous ROS and to their reduced increase following doxorubicin treatment in cyclin E-overexpressing derivatives compared with vector control cells (Figure 3 and Table 3). Reactive oxygen species are, in fact, general mediators of cell damage (Kehrer, 1993) and their generation is a prerequisite for the triggering of cell death by several stimuli, including growth factor withdrawal (Palazzotti et al, 1999) and activation of the tumour suppressor protein p53 (Polyak et al, 1997). Since both endogenous levels of ROS and intracellular oxidations induced by exogenous oxidants (H2O2 and doxorubicin) are diminished in Rat-E cells, these differences likely reflect an increased capacity of oxidant scavenging in cells overexpressing cyclin E. This hypothesis is supported by the observation that the protein expression and enzyme activity of the mitochondrial scavenger MnSOD, one of the major enzymes involved in the cellular defence against ROS, are upregulated (30% increase) in these cells compared with the corresponding controls (Figure 4). The observation that Rat-E did not display a reduced sensitivity to etoposide, which is a specific topoisomerase inhibitor, further supports our hypothesis that the increased resistance to doxorubicin is likely due to a reduced susceptibility of cells to the ROS-generating ability of the drug rather than to its inhibitory activity on topoisomerase II enzyme.
The mechanisms responsible for the increase of MnSOD expression in cyclin E-overexpressing cells remain unknown. However, it is of interest that some pools displayed a loss of expression of the exogenous cyclin E with prolonged serial passages that was associated with a decrease in the expression of MnSOD to the normal basal level (data not shown), thus further confirming the link between the two events. Moreover, we analysed by immunostaining the expression of the p53 protein in the Rat-V and Rat-E cells and found that both cell lines displayed a weak basal level of p53 nuclear staining that increased following doxorubicin treatment (data not shown). Thus, an involvement of the p53 protein in the MnSOD upregulation in cyclin E-overexpressing cells is unlikely. In preliminary experiments, we did not observe by Northern blot analysis any difference in the expression levels of MnSOD mRNA between cyclin E-overexpressing and vector cell lines (unpublished results). However, further studies are required to establish definitively the mechanism(s) responsible for the increase of MnSOD protein in cyclin E transfected cells, which might involve modification at the transcriptional, translational or post-translational levels.
We propose that the increased expression of MnSOD observed in Rat-E cells contributes to the resistance of these cells to doxorubicin. In fact, (a) we and others have previously reported that increased expression of MnSOD at levels comparable to those observed in Rat-E cells is associated with improved survival of tumour cells exposed to doxorubicin in vitro (Hirose et al, 1993; Pani et al, 2000) and an increased expression of SOD was previously reported in doxorubicin-resistant Friend leukaemic (about 24%) (Crescimanno et al, 1991) and breast cancer (Zyad et al, 1994) cell lines and in leukaemic patients (Watanabe, 1998); (b) compared with vector control cells, cyclin E-overexpressing Rat cells were also more resistant to Paraquat (IC50=370.9±9 vs 289.3±15.5 μ M) (data not shown), a superoxide-generating compound whose action is inhibited by SODs (Bus et al, 1974; Pani et al, 2000); thus further confirming the biological significance of the increase in MnSOD expression and activity observed in cyclin E-overexpressing cells compared with control cells. The finding that the CuZnSOD is not increased in Rat-E cells further confirms that MnSOD is selectively upregulated in cyclin E-overexpressing cells (Figure 4).
One of the major mechanisms of resistance to doxorubicin is due to the acquisition of the so-called multiple drug resistance (MDR) phenotype which, in most of the cases, depends on the expression of a transmembrane 170 kDa glycoprotein, called P-glycoprotein (Kartner et al, 1983). Cells that develop resistance through the MDR mechanism, however, simultaneously develop cross-resistance to several structurally unrelated natural products, including anthracyclines, vinka alkaloids, epipodophyllotoxins, taxanes and actinomycin-D (Kartner et al, 1983). Since the Rat-E cells only displayed an increased resistance to doxorubicin, but not to the vinca alkaloid vincristine, nor to paclitaxel, the acquisition of an MDR-phenotype in these cells is unlikely. However, while in light of the above consideration an involvement of MnSOD in Rat-E cell resistance to doxorubicin appears to be conceivable, the present results do not allow, at the moment, to exclude that other mechanisms, that is, involving ROS production and/or their detoxification or DNA repair, are also affected by cyclin E overexpression.
Although obtained in diploid immortalised rat fibroblasts, we believe that the results of the present study are instructive in revealing a potential new mechanism of resistance to doxorubicin in cancer cells. Regardless of the underlying molecular mechanisms, if it will be confirmed, the observed relation between the expression levels of cyclin E and cell sensitivity to doxorubicin might have important implications for the treatment of cancer, mainly breast cancer patients. In fact, doxorubicin is one of the most valuable anticancer drug in present day clinical use, being an integral part of the treatment of malignancies such as carcinoma of the breast, head and neck, thyroid and soft tissue sarcomas and leukaemias (DeVita et al, 2001). Moreover, on the other hand, increased expression of cyclin E is a common event in a variety of human malignancies (Donnellan and Chetty, 1999; Sandhu and Slingerland, 2000) and is believed to play an important role in cancer development and progression by causing cell growth deregulation and genomic instability (Bortner and Rosenberg, 1997; Spruck et al, 1999). Thus, it has been proposed that cyclin E may become a target for treatment of human cancers (Owa et al, 2001). Increased expression of cyclin E has been reported associated with poor prognosis in breast cancer patients (Nielsen et al, 1996; Porter et al, 1997). Since doxorubicin is included in most of the chemoterapeutic regimens for treatment of breast cancers, our results might also suggest that, at least in some cases, the reported relation between high cyclin E levels and poor prognosis in breast cancer patients might simply reflect the reduced responsivity of cyclin E-overexpressing tumours to doxorubicin (Keyomarsi et al, 1994; Nielsen et al, 1996). Further in vivo studies will be needed to address this point.
In conclusion, the results of the present study suggest that evaluation of cyclin E expression levels might help to select the most appropriate treatment for cancer patients, mainly breast cancer patients, by reserving anthracycline treatment to patients whose tumours do not overexpress this molecule. These results are in agreement with previous studies on the role of cyclin E and other cell cycle regulatory proteins (i.e. the CDK inhibitor p27Kip1) in determining the response of cells to cytotoxic agents and warrant further studies in this interesting area of cancer research (Croix et al, 1996; Smith and Seo, 2000; Yang et al, 2000).
Furthermore, the involvement of cyclin E and MnSOD in cell resistance to doxorubicin suggests that therapeuthical strategies (i.e. antisense or chemical inhibitors) targeting cyclin E/CDK (Sausville et al, 2000) or SOD activities (Huang et al, 2000) could also be tested as chemosensitising tools to anthracycline treatment in cyclin E-overexpressing tumours.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and one assay applicable to acrylamide gels. Anal Biochem 44: 276–287
Bortner DM, Rosenberg MP (1997) Induction of mammary gland hyperplasia and carcinomas in transgenic mice expressing human cyclin E. Mol Cell Biol 17: 453–459
Bus JS, Aust DD, Gibson JE (1974) Superoxide- and singlet oxygen-catalyzed lipid peroxidation as a possible mechanism for paraquat (methyl viologen) toxicity. Biochem Biophys Res Commun 58: 749–755
Bustamante J, Galleano M, Medrano EE, Boveris A (1990) Adriamicyn effects on hydroperoxide metabolism and growth of human breast tumor cells. Breast Cancer Res Treat 17: 145–153
Cacace AM, Guadagno SN, Krauss RS, Fabbro D, Weinstein IB (1993) The ipsilon isoform of protein kinase C is an oncogene when overexpressed in rat fibroblasts. Oncogene 8: 2095–2104
Clurman BE, Sheaff RJ, Thress K, Groudine M, Robertys JM (1996) Turnover of cyclin E by the ubiquitin–proteasome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev 10: 1979–1990
Crescimanno M, D'alessandro N, Armata MG, Toulmond S, Tapiero H (1991) Modulation of the antioxidant activities in dox-sensitive and -resistant Friend leukemia cells. Effect of doxorubicin. Anticancer Res 11: 901–903
Croix BS, Florenes VA, Rak JW, Flanagan M, Bhattacharya N, Slingerland JM, Kerbel RS (1996) Impact of the cyclin-dependent kinase inhibitor p27Kip1 on resistance of tumor cells to anticancer agents. Nature Med 2: 1204–1210
DeVita VT, Hellman S, Rosenberg SA (2001) Cancer: Principles and Practise of Oncology. Philadelphia: Lippincott Williams and Wilkins
Donnellan R, Chetty R (1999) Cyclin E in human cancers. FASEB J 13: 773–780
Doroshow JH (1983) Anthracycline antibiotics-stimulated superoxide, hydrogen peroxide and hydroxyl radical production by NADH dehydrogenase. Cancer Res 43: 4543–4551
Doroshow J (1986) Prevention of the doxorubicin induced killing of MCF-7 human breast cancer cells by oxygen radical scavengers and iron chelating agents. Biochem Biophys Res Commun 143: 309–315
Eymin B, Haugg M, Droin N, Sordet O, Dimanche-Boitrel M-T, Solary E (1999) p27Kip1 induces drug resistance by preventing apoptosis upstream of cytochrome c release and procaspase-3 activation in leukemic cells. Oncogene 18: 1411–1418
Fridovich I (1998) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64: 97–112
Friesen C, Fulda S, Debatin K-M (1999) Induction of CD95 ligand and apoptosis by doxorubicin is modulated by the redox state in chemosensitive- and drug-resistant tumor cells. Cell Death Differ 6: 471–480
Galeotti T, Borrello S, Masotti L (1990) Oxy-radical sources, scavenger systems and membrane damage in cancer cells. In Oxygen Radicals: Systemic Events and Disease Processes, Dass DK, Essman WB (eds) pp 129–148. Basel: Karger
Hirose K, Longo DL, Oppenhaim JJ, Matsuhima K (1993) Overexpression of mitochondrial manganese superoxide dismutase promotes the survival of tumor cells exposed to interleukin 1, tumor necrosis factor, selected anticancer drugs and ionizing radiation. FASEB J 7: 361–368
Hochhauser D, Schnieders B, Ercikan-Abali E, Gorlick R, Muise-Helmericks R, Li W-W, Fan J, Banerjee D, Bertino JR (1996) Effects of cyclin D1 overexpression on drug sensitivity in a human fibrosarcoma cell line. J Natl Cancer Inst 88: 1269–1275
Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W (2000) Superoxide dismutase as a target for the selective killing of cancer cells. Nature 407: 390–395
Kartner N, Riordan JR, Ling V (1983) Cell surface P-glycoprotein as associated with multidrug resistance in mammalian cell lines. Science 221: 1285–1288
Kasahara K, Fujiwara Y, Sugimoto Y, Nishio K, Tamura T, Matsuda T, Saijio N (1992) Determinants of the response to the DNA topoisomerase II inhibitors doxorubicin and etoposide in human lung cancer cell lines. J Natl Cancer Inst 84: 113–118
Kehrer J (1993) Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol 23: 21–48
Keyomarsi K, O'Leary N, Molnar G, Lees E, Fingert HJ, Pardee AB (1994) Cyclin E, a potential prognostic marker for breast cancer. Cancer Res 54: 380–385
Koff A, Cross F, Fisher A, Schumacher J, Leguellec K, Philippe M, Roberts JM (1991) Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell 66: 1217–1228
Nielsen NH, Arnerlov C, Emdin SO, Landberg G (1996) Cyclin E overexpression, a negative prognostic factor in breast cancer with strong correlation to oestrogen receptor status. Br J Cancer 74: 874–880
Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M (1995) Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol 15: 2612–2624
Owa T, Yoshino H, Yoshimatsu K, Nagasu T (2001) Cell cycle regulation in the G1 phase: a promising target for the development of new chemotherapeutic anticancer agents. Curr Med Chem 8: 1487–1503
Palazzotti B, Pani G, Colavitti R, De Leo ME, Bedogni B, Borrello S, Galeotti T (1999) Increased growth capacity of cervical-carcinoma cells overexpressing manganous superoxide dismutase. Int J Cancer 82: 145–150
Pani G, Bedogni B, Anzevino R, Colavitti R, Palazzotti B, Borrello S, Galeotti T (2000) Deregulated manganese superoxide dismutase expression and resistance to oxidative injury in p53-deficient cells. Cancer Res 60: 4654–4660
Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B (1997) A model for p53-induced apoptosis. Nature 389: 300–305
Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, Firpo EJ, Daling JR, Roberts JM (1997) Expression of cell-cycle regulators p27 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med 3: 222–225
Sandhu C, Slingerland J (2000) Deregulation of the cell cycle in cancer. Cancer Detect Prev 24: 107–118
Sausville EA, Johnson J, Alley M, Zaharevitz D, Senderowicz AM (2000) Inhibition of CDKs as a therapeutic modality. Ann NY Acad Sci 910: 207–221
Sgambato A, Ardito R, Faraglia B, Boninsegna A, Wolf FI, Cittadini A (2001) Resveratrol, a natural phenolic compound, inhibits cell proliferation and prevents oxidative DNA damage. Mut Res 496: 171–180
Sgambato A, Doki Y, Schieren I, Weinstein IB (1997) Effects of cyclin E overexpression on cell growth and response to TGF-β depend on cell context and p27Kip1 expression. Cell Growth Diff 8: 393–405
Sgambato A, Han EK-H, Zhou P, Schieren I, Weinstein IB (1996) Overexpression of cyclin E in the HC11 mouse mammary cell line is associated with growth inhibition and increased expression of p27Kip1. Cancer Res 56: 1389–1399
Sinha BK, Katki AG, Batist G, Cowan KH, Myers CE (1987) Adriamycin-stimulated hydroxyl radical formation in human breast tumor cells. Biochem Pharmacol 36: 793–796
Smith ML, Seo YR (2000) Sensitivity of cyclin E-overexpressing cells to cisplatin/taxol combinations. Anticancer Res 20: 2537–2539
Spruck CH, Won KA, Reed SI (1999) Deregulated cyclin E induces chromosome instability. Nature 401: 297–300
Watanabe N (1998) Endogenous tumor necrosis factor and doxorubicin sensitivity in leukemic patients. Leuk Lymphoma 30: 477–482
Yang Q, Sakurai T, Yoshimura G, Takashi Y, Suzuma T, Takami T, Umemura T, Nakamura Y, Nakamura M, Utsunomiya H, Mori I, Kakudo K (2000) Overexpression of p27 protein in human breast cancer correlates with in vitro resistance to doxorubicin and mitomycin C. Anticancer Res 20: 4319–4322
Zhu H, Bannenberg GL, Moldeus P, Shertzer HG (1994) Oxidation pathways for the intracellular probe 2′7′-dichlorofluorescein. Arch Toxicol 68: 582–587
Zyad A, Benard J, Tursz T, Clarke R, Chouaib S (1994) Resistance to TNF-alfa and adriamycin in the human breast cancer MCF-7 cell line: relationship to MDR1, MnSOD and TNF gene expression. Cancer Res 54: 825–831
Acknowledgements
‘Co-finanziamento MURST 2001’ and Università Cattolica (MURST 60%) sponsored grants to AC and Compagnia di San Paolo to AS. We are grateful to Dr IB Weinstein (Columbia University, New York, NY, USA) for helpful advices and for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Sgambato, A., Camerini, A., Pani, G. et al. Increased expression of cyclin E is associated with an increased resistance to doxorubicin in rat fibroblasts. Br J Cancer 88, 1956–1962 (2003). https://doi.org/10.1038/sj.bjc.6600970
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6600970
Keywords
This article is cited by
-
Targeted therapy against EGFR and VEGFR using ZD6474 enhances the therapeutic potential of UV-B phototherapy in breast cancer cells
Molecular Cancer (2013)
-
A unique RNA-directed nucleoside analog is cytotoxic to breast cancer cells and depletes cyclin E levels
Breast Cancer Research and Treatment (2010)