Abstract
Data from the Swedish Cancer Register 1987–1996 were used to examine seasonal trends in the diagnosis of cancer. For melanomas, prostate, breast and thyroid cancer there were clear seasonal variations with reductions in the number of cases reported during the summer and December that are likely to reflect mainly hospital delays.
Similar content being viewed by others
Main
There are reports of seasonal variations in the diagnosis and reporting of malignant melanomas or pigmented naevi (Swerdlow, 1985; Schwartz et al, 1987; Braun et al, 1994), lymphomas (Westerbeek et al, 1998), leukaemias (Badrinath et al, 1997; Eatough, 2002), breast cancer (Cohen et al, 1983; Kirkham et al, 1985; Mason et al, 1990; Ross et al, 1997; Dimitrov et al, 1998; Gao et al, 2001), and childhood cancers (Ross et al, 1999). Some of these variations in presentation have intriguing temporal patterns which reflect either biological phenomena or administrative differences in the likelihood of tumour detection and registration. We judged that further exploration of this issue is of interest.
Data from a nationwide Cancer Register provided an opportunity to examine seasonal trends in the diagnosis of cancer in Sweden by analysing all invasive cancers recorded between 1987 and 1996, a total of more than half a million incident cases.
Material and methods
The Swedish Cancer Registry was established in 1958 and covers the whole Swedish population (8.9 million), including foreign citizens and ethnic minorities with permanent residency.
Reporting of all new cancers and also certain benign tumours is mandatory, both in public and private care. Separate notifications should be filed by the clinician as well as by the pathologist/morphologist, which means that at least two reports per case are needed before registration. Furthermore, a new report must be issued if a substantial revision of the original diagnosis is made. The completeness of recording is estimated to be 95%. Approximately 97% of all recorded cases are morphologically verified (Swedish Cancer Registry, 2000).
Since 1985, reports are first sent to one of six regional Cancer Registries where patient data and tumours are recorded. For each calendar year, the regional registries submit the material to the National Cancer Registry for collation, merging of data and updating of the national files. A coordination group with representatives from the National Registry and each regional registry meet regularly to ascertain conformity in coding and data-processing routines.
The Cancer Registry publishes an annual report with the numbers of cancers, gender and age-specific and age-standardised incidence rates. Complete cancer incidence data for each year are released with about 2 years delay. At present, approximately 45 000 incident cases are recorded annually. Date of diagnosis is defined as the earliest examination date when the cancer was found (Swedish Cancer Registry, 2000).
The present study analysed tumours reported as definitely malignant neoplasms, a total of 555 948 incident tumours during the 10-year period 1987–1996. No national information was available on stage.
Statistical analyses
To detect any deviation from a uniform monthly distribution of the frequency of cancers by site throughout the year, a χ2 test for heterogeneity was used. In a subsequent approach, tumour reports from 1987 to 1996 were modelled by year and month to identify smooth trends during the years (Bacchetti, 1994). In our analysis, the temporal trend is a nuisance and was modelled initially both in continuous (linear and quadratic terms) and in a completely unstructured way using indicator variables. A better approach is to use parametric natural cubic B splines with evenly spaced knots (De Boor, 1978).
Monthly effects were modelled generally as M(j)=M(j+12) and M(1)=0 for 11 free parameters, or with the following reduced model:

where c(j) is the calendar month (i.e., 1 if j is January of any year, 2 if j is February, etc.), the σ's model any truly seasonal patterns, τ is the size of the jump between December and January and models any linear trend over the calendar year, and γ is a general summer effect (July and August) beyond that captured by the linear trend and the seasonal terms (the summer spike). The model uses only six parameters, instead of the 11 needed for completely arbitrary month effects, separating seasonal components from other influences.
Cancer incidence was modelled with both negative binomial regression to adjust for overdispersion and lack of fit in Poisson's regression. For each of the four types of cancers that deviated from a uniform temporal distribution, the parameter estimates were adjusted by gender, age and region. Model estimation and fitting was performed using STATA 7.0 (Stata Corp, 2000).
Results
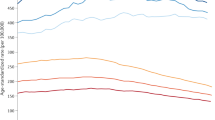
We first examined the combined monthly distribution for all cancer sites (ICD-7: 140–209). Malignant melanomas, prostate, breast and thyroid cancers were the only sites for which a deviation from a uniform distribution throughout the year could be detected (Table 1). For melanomas, the number of new cases reported was lowest during the winter months while monthly frequencies peaked at two different points: in May–June and September–October. For prostate, breast and thyroid, there was a clear decrease in the frequency of new cases diagnosed during the summer months, followed by a transient increase in subsequent months. The highest mean number of cases was reported in October and November for prostate and breast cancer, respectively. For all four cancer types, there was a decrease in December followed by an increase in January and February. Seasonal trends (based on 3-months moving averages) are illustrated in Figure 1.
In a second analytic step, monthly counts from the whole period of observation were modelled in terms of secular trends and short-term effects to identify smooth trends over the year, adjusted by gender, age and region. Table 2 shows a comparison of the relative seasonal changes in registration. The most pronounced summer season decrease was seen for melanomas (−28.4%), while thyroid cancer had the highest relative increase between December and January (+27.6%). When assessed by age at diagnosis, year of diagnosis, or by health-care region, the observed patterns did not change (data not shown).
Discussion
For the great majority of cancers, we found a uniform seasonal distribution of detection. However, for melanomas, prostate, breast and thyroid cancer there were reductions in the number of cases diagnosed during the summer and the month of December.
Melanoma
For melanomas, our results broadly corroborate findings in at least three earlier studies that found evidence of seasonal patterns of detection (Swerdlow, 1985; Schwartz et al, 1987; Braun et al, 1994). At northern latitudes such as in Sweden, an early (May–June) peak could reflect an increased patient awareness and self-detection of abnormal naevi coinciding with the change to summer clothing. The postsummer (September–October) peak may, however, be a result of a promoting effect of sun exposure, resulting in signs and symptoms of malignant changes in pre-existing naevi.
Breast cancer
The sharp decrease in the number of new cases of breast cancers recorded in June and July is likely to reflect mainly the reduced activity of mammography screening programmes during the summer months. These programmes were introduced in Sweden in the late 1980s. By 1993, more than 90% of women of eligible ages (generally 50–74 years) were invited to regional screening programmes. However, in a separate analyses of cases recorded during 1988 and earlier, a similar reduction during the summer was found. Patients' delay may also contribute to the summer decrease. Signs and symptoms are disregarded and not attended to until after vacation time. The results of earlier studies of possible seasonality of breast cancer have varied (Cohen et al, 1983; Kirkham et al, 1985; Mason et al, 1990; Ross et al, 1999; Gao et al, 2001), but none have found a similar pronounced summer decrease.
Prostate and thyroid cancer
To our knowledge, no previous study has investigated seasonal variations in the diagnosis of prostate and thyroid cancer. As in the case of breast cancer, our findings of a decrease during the summer are likely to reflect a reduced capacity to ascertain suspected cases within the health-care system.
Our study is the largest reported on this subject so far. It was based on national cancer register data of high validity to which all tumours are reported in a country with a uniform national public health-care system. Given the consistency of our findings when assessed by region or time period, chance is an unlikely explanation for the observed patterns. It also appears unlikely that our results can be explained by systematically delayed reporting or data entry by season at the regional registries. If this had occurred, seasonal variations would have been observed for a broader range of cancers.
In conclusion, our findings of seasonal variations in the diagnosis of melanomas, thyroid, breast and prostate cancer are likely to reflect both patient and hospital delay. A common clinical feature of these four cancers is that they often present with mild, nonacute symptoms, allowing delay of self-referral. It is also conceivable that the lower diagnostic intensity during summer and around the turn of the year reflects a reduced capacity to evaluate patients with suspected cancers. For melanomas, however, the bimodal diagnostic pattern would be in keeping with seasonal exposure to solar radiation.
Overall, the observed variations in detection intensity appear unlikely to have clinical implications or influence survival negatively, since very rapid progression is rare in these tumours. However, any seasonally related delay between first visit, referral and final diagnosis of cancer because of a reduced capacity within the health-care system may cause anxiety among patients. Further efforts to minimise delays are important, for example, by implementing quality guidelines where patients with a suspected cancer are guaranteed to be evaluated, diagnosed and treated within a stipulated time period.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bacchetti P (1994) Seasonal and other short-term influences on United States AIDS incidence. Stat Med 13: 1921–1931
Badrinath P, Day NE, Stockton D (1997) Seasonality in the diagnosis of acute lymphocytic leukaemia [see comments]. Br J Cancer 75: 1711–1713
Braun MM, Tucker MA, Devesa SS, Hoover RN (1994) Seasonal variation in frequency of diagnosis of cutaneous malignant melanoma. Melanoma Res 4: 235–241
Cohen P, Wax Y, Modan B (1983) Seasonality in the occurrence of breast cancer. Cancer Res 43: 892–896
De Boor CA (1978) A Practical Guide to Splines. New York: Springer-Verlag
Dimitrov BD, Shangova-Grigoriadi S, Grigoriadis ED (1998) Cyclicity in variations of incidence rates for breast cancer in different countries. Folia Med (Plovdiv) 40: 66–71
Eatough JP (2002) Evidence of seasonality in the diagnosis of monocytic leukaemia. Br J Cancer 87: 509–510
Gao F, Machin D, Khoo K-S, Ng E-H (2001) Seasonal variation in breast cancer diagnosis in Singapore. Br J Cancer 84: 1185–1187
Kirkham N, Machin D, Cotton DW, Pike JM (1985) Seasonality and breast cancer. Eur J Surg Oncol 11: 143–146
Mason BH, Holdaway IM, Stewart AW, Neave LM, Kay RG (1990) Season of initial discovery of tumour as an independent variable predicting survival in breast cancer [see comments]. Br J Cancer 61: 137–141
Ross JA, Severson RK, Davis S, Stanford JL, Potter JD (1997) Seasonal trends in the self-detection of breast cancer: indications from the Cancer and Steroid Hormone (CASH) study. Breast Cancer Res Treat 42: 187–192
Ross JA, Severson RK, Swensen AR, Pollock BH, Gurney JG, Robison LL (1999) Seasonal variations in the diagnosis of childhood cancer in the United States. Br J Cancer 81: 549–553
Schwartz SM, Armstrong BK, Weiss NS (1987) Seasonal variation in the incidence of cutaneous malignant melanoma: an analysis by body site and histologic type. Am J Epidemiol 126: 104–111
Swedish Cancer Registry (2000) Cancer Incidence in Sweden 1998. Stockholm: Socialstyrelsen
Swerdlow AJ (1985) Seasonality of presentation of cutaneous melanoma, squamous cell cancer and basal cell cancer in the Oxford Region. Br J Cancer 52: 893–900
Westerbeek RM, Blair V, Eden OB, Kelsey AM, Stevens RF, Will AM, Taylor GM, Birch JM (1998) Seasonal variations in the onset of childhood leukaemia and lymphoma. Br J Cancer 78: 119–124
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Lambe, M., Blomqvist, P. & Bellocco, R. Seasonal variation in the diagnosis of cancer: a study based on national cancer registration in Sweden. Br J Cancer 88, 1358–1360 (2003). https://doi.org/10.1038/sj.bjc.6600901
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6600901
Keywords
This article is cited by
-
Spatial identification of potential health hazards: a systematic areal search approach
International Journal of Health Geographics (2017)
-
Data-driven discovery of seasonally linked diseases from an Electronic Health Records system
BMC Bioinformatics (2014)
-
Seasonal variation in the presentation of thyroid cancer in the USA: an analysis of the Surveillance, Epidemiology and End Results Registry
Cancer Causes & Control (2014)
-
First description of seasonality of birth and diagnosis amongst teenagers and young adults with cancer aged 15–24 years in England, 1996–2005
BMC Cancer (2013)
-
Global breast cancer seasonality
Breast Cancer Research and Treatment (2010)