Abstract
Data sources
Cochrane Oral Health's Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), Medline, Embase, CINAHL, EBSCO (Cumulative Index to Nursing and Allied Health Literature, LILACS, BIREME, Virtual Health Library (Latin American and Caribbean Health Science Information database), Zetoc Conference Proceedings, the US National Institutes of Health Ongoing Trials Register, (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform for ongoing trials. No restrictions were placed on the language or date of publication when searching the electronic databases.
Study selection
The review included randomised controlled trials, irrespective of their language of publication or publication status. Participants could be outpatients or inpatients. The review included trials comparing any pharmacological agent regimen, prescribed prophylactically for salivary gland dysfunction prior to or during radiotherapy, with placebo, no intervention or an alternative pharmacological intervention. Comparisons of radiation techniques were excluded.
Data extraction and synthesis
Standard Cochrane methodological processes were followed.
Results
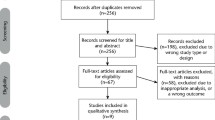
Thirty-nine studies that randomised 3520 participants were included; the number of participants analysed varied by outcome and time point. The studies were ordered into 14 separate comparisons with meta-analysis only being possible in three of those. We found low quality evidence to show that amifostine, when compared to a placebo or no treatment control, might reduce the risk of moderate to severe xerostomia (grade 2 or higher on a 0 to 4 scale) at the end of radiotherapy (risk ratio (RR) 0.35, 95% confidence interval (CI) 0.19 to 0.67; P = 0.001, three studies, 119 participants), and up to three months after radiotherapy (RR 0.66, 95% CI 0.48 to 0.92; P = 0.01, five studies, 687 participants), but there is insufficient evidence that the effect is sustained up to 12 months after radiotherapy (RR 0.70, 95% CI 0.40 to 1.23; P = 0.21, seven studies, 682 participants). We found very low quality evidence that amifostine increased unstimulated salivary flow rate up to 12 months after radiotherapy, both in terms of mg of saliva per five minutes (mean difference (MD) 0.32, 95% CI 0.09 to 0.55; P = 0.006, one study, 27 participants), and incidence of producing greater than 0.1 g of saliva over five minutes (RR 1.45, 95%CI 1.13 to 1.86; P = 0.004, one study, 175 participants).
However, there was insufficient evidence to show a difference when looking at stimulated salivary flow rates. There was insufficient (very low quality) evidence to show that amifostine compromised the effects of cancer treatment when looking at survival measures. There was some very low quality evidence of a small benefit for amifostine in terms of quality of life (ten-point scale) at 12 months after radiotherapy (MD 0.70, 95% CI 0.20 to 1.20; P = 0.006, one study, 180 participants), but insufficient evidence at the end of and up to three-month post radiotherapy. A further study showed no evidence of a difference at 6, 12, 18 and 24-month post radiotherapy.
There was low quality evidence that amifostine is associated with increases in: vomiting (RR 4.90, 95% CI 2.87 to 8.38; P < 0.00001, five studies, 601 participants); hypotension (RR 9.20, 95% CI 2.84 to 29.83; P = 0.0002, three studies, 376 participants); nausea (RR 2.60, 95% CI 1.81 to 3.74; P < 0.00001, four studies, 556 participants); and allergic response (RR 7.51, 95% CI 1.40 to 40.39; P = 0.02, three studies, 524 participants).
The authors founded insufficient evidence (that was of very low quality) to determine whether or not pilocarpine performed better or worse than a placebo or no treatment control for the outcomes: xerostomia, salivary flow rate, survival and quality of life. There was some low quality evidence that pilocarpine was associated with an increase in sweating (RR 2.98, 95% CI 1.43 to 6.22; P = 0.004, five studies, 389 participants).
The authors found insufficient evidence to determine whether or not palifermin performed better or worse than placebo for: xerostomia (low quality); survival (moderate quality); and any adverse effects. There was also insufficient evidence to determine the effects of the following interventions: biperiden plus pilocarpine, Chinese medicines, bethanechol, artificial saliva, selenium, antiseptic mouthrinse, antimicrobial lozenge, polaprezinc, azulene rinse and Venalot Depot (coumarin plus troxerutin).
Conclusions
There is some low quality evidence to suggest that amifostine prevents the feeling of dry mouth in people receiving radiotherapy to the head and neck (with or without chemotherapy) in the short- (end of radiotherapy) to medium-term (three-month post radiotherapy). However, it is less clear whether or not this effect is sustained to 12-month post radiotherapy. The benefits of amifostine should be weighed against its high cost and side effects. There was insufficient evidence to show that any other intervention is beneficial.
Similar content being viewed by others
Commentary
Saliva acts and is needed as a protectant in the oral cavity. Produced by the submandibular, sublingual and parotid gland, saliva provides an important buffering capacity to negate the effect of acidic foods in the prevention of caries, acts as a lubricant for the soft tissue and is the first step in digestion. One may imagine what a large impact it would make on a patient's quality of life when there is decreased production. Exposure of salivary glands to irradiation leads to hyposalivation resulting in xerostomia.1
Salivary gland dysfunction is common in patients undergoing radiotherapy to the head and neck. Most radiation therapy (RT) toxicity can be divided into acute toxicity, which is largely unavoidable but transient, and late toxicity, which can be minimised but is generally long lasting and in some instances permanent.2
The extent of damage to the glands is dependent on dose and length of time. Conformal RT, which shapes the radiation to the shape of the tumor, works on minimising impact to adjacent tissue and has been known to lessen xerostomia. Clinical symptoms are still present and can easily impede the patient's quality of life significantly. Normal day-to-day functions can become painful due to the mucosa becoming so dry that movement of the mouth for speech can be painful. Inability to swallow or eat a meal due to lack of saliva can lead to the patient avoiding eating and loss of weight. Typically, there are many options on the market that have been recommended with limited improvement clinically for patients.
Pilocarpine has long been used as a salivary gland stimulant. It has been studied as a means of stimulating saliva in residual salivary gland tissue post-radiation. A systematic review of the literature that included 11 randomised trials concluded that oral pilocarpine could be not be recommended to prevent xerostomia in patients receiving RT for head and neck cancer.3
Bethenacol has also been studied and unlike pilocarpine, studies suggest it may improve salivary flow and decrease patients' complaints of xerostomia. Unfortunately, there is lack of data supporting long-term impact.
Palifermin has been studied in the treatment of mucositis which may develop as a result of chemoradiation but is seldom used in routine practice due to its considerable cost and minimal benefit. There was insufficient evidence as to its benefit for xerostomia.
The methodology for this Cochrane review was conducted appropriately. The types of studies used were randomised controlled trials and controlled trials of parallel design irrespective of language or publication status. Gender, age or ethnicity was not used for exclusion. Participants were scheduled to receive radiotherapy alone to the head and neck region or in combination with chemotherapy and could be inpatient or outpatient. Those interventions assessed were any pharmacological agent prescribed prior to or during radiotherapy for preventive reasons by means of any route, any dose and any length of time. This study excluded radiation techniques. These were compared to control groups where no preventive intervention, placebo or other pharmacological preventive measure for salivary gland dysfunctionwas used. Outcome measures were based on long-term effects and were collected at the end of radiotherapy, except in the case of adverse effects. Primary outcome was salivary gland dysfunction indicated by xerostomia (visual analogue scales or verbal rating scales) and salivary flow rates. Secondary outcomes measured were adverse effects, survival data, other oral signs/symptoms, quality of life, patient satisfaction and cost data. The pharmacological comparisons used were: pilocarpine, amifostine (single dose, comparison of doses, different routes), palifermin, biperiden, bethanechol, bethanechol vs artificial saliva, selenium, antiseptic mouthrinse, antimicrobial lozenge, polaprezinc vs asulene oral rinse and Venalot Depot. Despite the fact that there were 39 studies, the Cochrane reviewers found the evidence was insufficient to indicate much promise for effective prophylactic treatment in salivary gland dysfunction. This was mostly due to the inconsistencies in outcome reporting and timing.
The results in this extensive review concluded that there is low evidence favouring amifostine compared to placebo to improve xerostomia, salivary flow and quality of life. However the prescription is costly and not free of important side effects such as nausea, vomiting and hypotension. All of the evidence for the remaining interventions is insufficient.
The results of this review, as well as other reviews, indicate there is a need for further high quality research. Long-term effects of amifostine should be studied along with palifermin. The challenge remains for the clinician to find a cost-effective intervention that works for short and long term benefit to maintain quality of life.
References
Kumar V . Artificial salivary glands - an innovative treatment for salivary gland dysfunction. Indian J Stomatol 2011; 2:172–174.
Amdur RJ . Management and prevention of complications during initial treatment of head and neck cancer. In Posner M, Bockstein B, Brizel D, Deschler D (eds). UpToDate. UpToDate, Waltham, MA; 2014. https://www.uptodate.com/contents/management-and-prevention-of-complications-during-initial-treatment-of-head-and-neck-cancer (accessed March 2018)
Jensen SB, Pedersen AM, Vissink A, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer 2010; 18:1061–1079.
Author information
Authors and Affiliations
Additional information
Address for correspondence: Luisa Fernandez Mauleffinch, Managing Editor, Cochrane Oral Health Group, School of Dentistry, The University of Manchester, JR Moore Building, Oxford Road, Manchester, M13 9PL, UK. E-mail: luisa.fernandez@manchester.ac.uk
Riley P, Glenny AM, Hua F, Worthington HV. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database Syst Rev 2017; 7: Art. No.: CD012744. DOI: 10.1002/14651858.CD012744.
This paper is based on a Cochrane Review published in the Cochrane Library 2017, issue 7 (see www.thecochranelibrary.com for information). Cochrane Reviews are regularly updated as new evidence emerges and in response to feedback, and the Cochrane Library should be consulted for the most recent version of the review.
Rights and permissions
About this article
Cite this article
Ferraiolo, D., Veitz-Keenan, A. Insufficient evidence for interventions to prevent dry mouth and salivary gland dysfunction post head and neck radiotherapy. Evid Based Dent 19, 30–31 (2018). https://doi.org/10.1038/sj.ebd.6401295
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ebd.6401295
This article is cited by
-
Gingival-derived mesenchymal stem cell therapy regenerated the radiated salivary glands: functional and histological evidence in murine model
Stem Cell Research & Therapy (2024)
-
Biofabrication, biochemical profiling, and in vitro applications of salivary gland decellularized matrices via magnetic bioassembly platforms
Cell and Tissue Research (2023)