Abstract
Release of apoptogenic factors into the cytosol including cytochrome c is triggering the execution phase of apoptosis through activation of cytoplasmic effector caspases. How loss of function of the electron transport chain can be reconciled with an adequate energy supply necessary for executing the apoptotic program was studied in granulosa cell (GC) sheets cultured up to 72 h without gonadotrophic support. Cytochrome c was localized ultrastructurally by oxidation of diaminobenzidine tetrahydrochloride both in living and fixed cells. In uncultured GC sheets all cells show staining over their entire mitochondrial population. In 72 h cultured sheets in the absence of FSH pre-apoptotic GC's display two subsets of mitochondria: normal sized stained mitochondria and small orthodox mitochondria without demonstrable cytochrome function. Apoptotic cells contain several mitochondria with preservation of respiratory function besides unstained orthodox mitochondria. The cytochrome c containing mitochondria typically display dilated intracristal spaces, a mitochondrial conformation related to increased ATP production. Cytochrome c release was confirmed by Western blotting. In 72 h cultures supplemented with FSH, GC's displayed staining over their entire mitochondrial population. In cultures lacking FSH, but partially protected from apoptosis through caspase inhibition, the cytochrome c release was not inhibited. Thus in the present studied model dysfunction of only a subset of mitochondria is instrumental to initiate the apoptotic program while a functional electron transport chain is maintained until the degradation phase in a subset of respiring mitochondria.
Similar content being viewed by others
Introduction
Preservation of morphologically intact mitochondria has been considered a hallmark of apoptotic cell death.1 However, mitochondria are known to be implicated in apoptosis since the localisation of the anti-apoptotic Bcl-2 gene product at the outer mitochondrial membrane.2 Newmeyer and colleagues showed that it was the mitochondrial fraction of the Xenopus oocyte extract which induced apoptosis.3 At the other it was reported that in cell-free systems nuclear apoptosis was dependent on the presence of ATP.4 Intracellular ATP levels have been implicated both in vitro and in vivo as a determinant of the cell's decision to die by apoptosis or necrosis.5,6,7 In discordance with the need for ATP are the studies showing that an early common event is the dissipation of the transmembrane potential with uncoupling of the electron transport from ATP production.8 A link between mitochondrial events and the activation of the proteolytic caspase cascade is cytochrome c efflux from the mitochondria,9 although the mechanism by which cytochrome c is able to escape from the mitochondria is still a matter of debate.10,11 In any case, the efflux of cytochrome c is under Bcl-2 control.12,13,14 The discrepancy between mitochondrial dysfunction and the need for ATP to execute the apoptotic program can only be resolved if either the mitochondrial changes are only involving a subset of mitochondria; or the escaping cyt c is only a small fraction of mitochondrial cyt c, not impairing the electron transport chain; or the energy supply is maintained by glycolysis. The present study addresses the following questions: (1) are mitochondrial changes (as revealed by cytochrome function) an early instrumental event during gonadotropin-withdrawal elicited apoptosis in the granulosa cell sheet model?; (2) are all mitochondria equally involved in the cytochrome c efflux?; and (3) is cytochrome c release an event upstream of caspase activation?
Previous studies showed that apoptosis is initiated in the granulosa cell sheet model after 24 h of serum-free culture in the absence of gondadotrophic support, increasing thereafter with a maximum at 72 h.15,16
Results
Cytochrome oxidase activity
The diaminobenzidine technique used in the present study localizes cytochrome c in individual mitochondria, but requires cytochrome oxidase activity to oxidize the reduced cytochrome c by means of O2 with the generation of H2O as shown in Figure 1. Because of the implication of both cytochrome c and cytochrome oxidase, loss of either enzyme can cause a loss of staining. Therefore it was crucial to determine cytochrome oxidase activities in 72 h cultures in the absence/presence of FSH, i.e. in apoptotic cultures and cultures in which apoptosis was inhibited. We found no significant difference between cytochrome oxidase activities expressed in ng/min/mg protein in 72 h cultures in the absence/presence of FSH (five independent experiments; see Table 1).
Light microscopical observations
In uncultured GC sheets mitochondrial staining is intense and the pattern is uniform throughout the sheet. In 72 h cultured GC sheets in the absence of FSH the overall staining decreases and is not uniform throughout the cell layer: i.e. some cells with normal nuclei are surrounded by very few stained mitochondria, while other adjacent cells display a sizable number of reactive mitochondria. At this culture stage GC-sheets display an A.I. of 40%±9 S.E.M. as calculated on DAPI stained sheets in four independent experiments. A number of apoptotic cells identified by their condensed chromatin contain reactive organelles. In 72 h FSH-supplemented cultures the apoptotic process is inhibited significantly (A.I<5%) and the heterogeneous pattern found in the serum-free cultures was absent: i.e. all normal cells showed a similar number of stained organelles (Figure 2A,B).
Light microscopy (interference contrast); cytochrome c localization (brown) in 72 h living GC sheet, methylgreen counterstaining. (A) FSH supplemented GC sheet. All GC's contain a sizable number of reactive mitochondria. Rare apoptotic nuclei can be found under FSH supplementation (arrowhead). (B) GC sheet cultured in the absence of FSH; normal nuclei faintly stained are indicated by asterisks. In one apoptotic cell with condensed chromatin (green mass) several reactive mitochondria are seen (arrowhead), while other apoptotic bodies are devoid of stained mitochondria. Remark the presence of some normal nuclei surrounded by few or no reactive organelles. Bars: 10 μm
Granulosa cell sheets cultured for 48 h in the absence of FSH (AI of circa 20%) were partially protected from apoptosis through caspase inhibition (50 μm zVAD-fmk). Comparison between zVAD-fmk treated and untreated cultures both in the absence of FSH yielded a significant inhibition of the AI. In 24 h cultures zVAD-fmk treatment reduced the apoptotic index by 71%±11 S.E.M. (see Figure 3) while at 48 h culture a reduction of 60% was calculated (data not shown). In the 48 h cultures under zVAD treatment the cytochrome c staining was similar to untreated cultures. Again a heterogeneous pattern of staining in normal non apoptotic cells was apparent, with some cells containing very few reactive organelles adjacent to cells containing a sizable number of reactive organelles (data not shown).
Ultrastructural observations
In uncultured GC sheets the mitochondria appeared in the orthodox conformation; the reaction product was localized in the intermembrane space and at the intracristal spaces of all mitochondria (Figure 4).
Electron microscopy; cytochrome c localization (osmiophilic reaction product) in uncultured GC sheets. Mitochondria display the orthodox configuration; all are stained, reaction product confined to intracristal spaces and intermembrane space. Some mitochondria are cut tangentially and therefore do not show a sharply delineated reaction product (arrow); BM: basement membrane. Bar: 0.5 μm
In 72 h cultures in the absence of FSH, a fraction of cells with normal nuclear morphology showed a number of unreactive mitochondria (Figure 5A). Importantly these unreactive mitochondria displayed an orthodox configuration of their membranes and matrix, often had elongated forms and seemed smaller than the reactive organelles. Discontinuities of outer mitochondrial membranes were not identified.
Electron microscopy; cytochrome c localization (osmiophilic reaction product) in 72 h living GC sheet cultured in the absence of FSH. (A) Pre-apoptotic cells with normal nuclear morphology contain two subsets of mitochondria: small unreactive mitochondria with orthodox configuration (arrowheads) and normal sized reactive mitochondria (arrows). (B) Apoptotic cell with dense cytoplasm and condensed chromatin mass. Reaction product is prominent in dilated intracristal spaces of two reactive mitochondria, while four mitochondria with orthodox configuration are unreactive (arrowhead). Bars: 1 μm
Sections through apoptotic cells showed several stained organelles besides a number of unstained but ultrastructurally normal small mitochondria Figure 5B). Typically the reaction product was most obvious in the intracristal space which was enlarged (intracristal swelling or ballooning) as compared to unreactive organelles. In some instances the reaction is confined to the intracristal spaces with no reactivity at all in the intermembrane space.
In the 72 h FSH-supplemented cultures, where the apoptotic process was inhibited, all mitochondria in normal cells behaved similarly, i.e. they were reactive.
In 48 h cultured GC sheets in the absence of FSH but treated during the last 24 h with the pancaspase inhibitor zVAD-fmk the cytochrome c release seemed unaffected. Again the two subsets of mitochondria were recognized: small unreactive mitochondria besides larger staining mitochondria with dilatated intracristal spaces (Figure 6). Importantly, now the mitochondria with enlarged intracristal spaces were not only present in clear-cut apoptotic cells, but also in the population of ultrastructurally normal cells. Typically apoptotic nuclear morphology in zVAD-fmk treated cultures was aberrant: many nuclear fragments remained enclosed within one cytoplasm; i.e. fragmentation of apoptotic cells in apoptotic bodies seemed inhibited.
Electron microscopy; cytochrome c localization (osmiophilic reaction product) in 48 h GC sheet cultured in the absence of FSH under zVAD-fmk treatment. In the remaining apoptotic cells identical activation of mitochondria with preserved cytochrome function (reaction product in dilated cristal spaces (arrows)) could be observed. These activated mitochondria were also found in non-apoptotic cells (not shown). Note apoptotic nuclear fragments (A). Bar: 1 μm
Incubations performed on prefixed tissue (data not shown) yielded similar results as those performed on living tissue (Figures 2A,B, 4, 5A,B and 6).
Biochemical confirmation of cytochrome c release
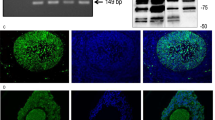
In order to confirm that the unreactive mitochondria in pre-apoptotic and apoptotic cells (Figure 5A,B) have released cytochrome c, cytosolic extracts of 72 h cultured granulosa cell sheets were prepared under conditions that keep mitochondria intact and compared to extracts of the pellet fraction in which mitochondrial cyt c is detected. This procedure was used for apoptotic cultures (72 h cultures in the absence of FSH) and for controls (uncultured granulosa cell sheets). Cytosol from the control cultures did not contain any detectable cytosolic cytochrome c protein (Figure 7, lane 3) indicating that the procedure did not induce mitochondrial contamination. In contrast, cytosolic cytochrome c accumulated significantly in the 72 h GC sheets cultures in the absence of FSH (Figure 7, lane 4).
Cytochrome c is released in the 72 h CC sheets cultured in the absence of FSH and not in control (uncultured) GC sheets; compare lanes 3 and 4. Cytochrome c present in the cytosol was determined after cell lysis in the presence of 0.075% digitonin. The amount of cytochrome c that is left in the pellet fraction (released by 1% NP 40) represent the mitochondrial cytochrome c present in the cells (lanes 1,2)
Morphometrical analysis
In order to validate the impression that the negative subset of mitochrondria were smaller as compared to the reactive ones, the mean square of the two subsets of mitochondria was calculated.
From this analysis it appears that the negative mitochondria are significantly smaller as compared to the reactive subset (see Table 2).
Discussion
The implication of cytochrome c in apoptosis is studied by various techniques, but most approaches fail to provide data on individual mitochondria in intact cells. Indeed, it was reported that ‘it is not known until now whether all mitochondria within each cell are affected to a similar extent’.17 Several investigators have already suggested the possibility of mitochondrial heterogeneity with respect to cytochrome c efflux or mitochondrial dysfunction as revealed by dissipation of transmembrane potential or permeability transition.18,19,20,21,22,23,24 In two landmark papers cytochrome c release was shown by subcellular fractionation.12,13 Other groups demonstrated the cell-specific induction of apoptosis by microinjection of cytochrome c in cells expressing caspase 324,25 or localized cytochrome c in the cytoplasm by immunofluorescence.14,26 By the latter technique, cells with a mixed punctuate/diffuse pattern could be observed very rarely.27 As far as we know cytochrome c was not yet localized in individual mitochondria of apoptotic cells by immunogold technique. Data concerning the extent of cytochrome c release or the compartment within the mitochondria from which cytochrome c is released are scarce. It was reported that 40% of total cytochrome c is released in a cyclosporin A-insensitive manner from brain mitochondria induced by calcium overload, however this study did not resolve the issue whether all mitochondria of a given cell are equally involved in this efflux.28
In Bax expressing yeast cells cytochrome c release was accompanied by a decrease in the amount of cytochrome oxidase which could be prevented by co-expression of Bcl-XL.29 Several studies assaying cytochrome c in the cytosol have also assayed cytochrome oxidase to exclude mitochondrial contamination; efflux of cytochrome oxidase was never shown.17,30 Surprisingly high levels of cytochrome oxidase activity in mitochondria were observed cytochemically in pre-apoptotic and apoptotic hepatocytes.31 We assayed cytochrome oxidase in 72 h cultured sheets in the absence/presence of FSH. Absence of a significant difference suggests that the appearance of an unstained subset of mitochondria during apoptosis is not due to a decrease in cytochrome oxidase activity but to either inactivation of cytochrome c in situ or to the efflux of cytochrome c. The latter proposal being confirmed by the Western blots showing indeed cytosolic cytochrome c in the 72 h serum-free cultures. To be rigorous we should consider the possibility that the small non respiring mitochondria are defective at the biosynthetic level. Indeed, accumulation of newly synthesised mitochondria with impaired function was reported in Colo-205 cells treated with herbimycin A. These mitochondria have a dissipated mitochondrial transmembrane potential, they are electron dense and have a reduced volume.32 In this case the cytosolic cytochrome c detected in the 72 h cultured GC sheets would be derived from the positively staining subset. In any case the appearance of a subset of non-respiring but intact mitochondria is tightly linked to the apoptotic process as evidenced by the absence of negative mitochondria in 72 h FSH-supplemented cultures.
In two complementary studies it was shown that a reversible transition of mitochondrial morphology from an orthodox conformation to one with dilated intracristal spaces is associated with transition from a resting energy state to a state of increased oxidative phosphorylation.33,34 In the apoptotic cells we found two subsets of mitochondria, and in addition the respiring mitochondria display now the conformation related to increased ATP generation. Hackenbrock called this the ‘condensed conformation’, but also stresses that a more specific sign of stimulated mitochondria is the intracristal dilatation.34 In fact a previous study on etoposide treated THP.1 cells showed intracristal dilatation in the ‘ultracondensed’ mitochondria.17 Moreover, besides the altered mitochondria, these authors reported casually the persistence of mitochondria with normal ultrastructural configuration. The presence of a subset of respiring mitochondria until late in the apoptotic process is in agreement with the late drop in ATP measured during apoptosis.26
By morphometrical analysis we have shown that the cytochrome c depleted mitochondria are smaller than the cytochrome c containing subset. Others have reported the shrinkage of mitochondria after cytochrome c depletion in NGF-deprived sympathetic neurons.35 Our subset of non-respiring mitochondria do not show ultrastructural features of dysfunction: they do not show discontinuities of the outer mitochondrial membrane,36 they are not swollen and show the orthodox conformation.
The maintained cytochrome c release and the ultrastructural changes in mitochondria of the caspase-inhibited cultures suggest that the cyt c release is upstream of caspase activation in our model system, as is the case in most apoptotic systems.12,26,37 Moreover, it suggests that the ultrastructural changes (intracristal dilatation) are not a late byproduct of caspase activation. This finding contrasts with the ‘ultracondensed’ mitochondria reported by Zhuang and colleagues; here the ultrastructural change (zVAD-fmk inhibitable) was clearly an event occurring downstream of the activation of caspases.17
The aberrant morphology of the apoptotic cells under zVAD-fmk protection needs to be further investigated. Although the latter suggests a role for caspases in fragmentation of apoptotic cells, zVAD-fmk mediated inhibition of non-caspases (as for example cathepsin B)38 might also be responsible for this observation.
In conclusion we have shown that during apoptosis in the granulosa cell sheet model a subset of mitochondria retains cytochrome c function and hence the possibility for ATP production up to the stage of chromatin condensation and fragmentation. A second subset of mitochondria is non respiring; their normal ultrastructural appearance points to a selective protein channel as the mechanism for cytochrome c efflux.
Materials and Methods
Isolation and culture of GC sheets
GC sheets were prepared from ovarian follicles of adult regularly laying Japanese quail (Coturnix coturnix japonica). The animals were reared under continuous artificial illumination, with food and water ad libitum. The monolayered granulosa layer of the largest preovulatory follicle (F1) was isolated from the surrounding thecal covering as previously described.39 This method provides large sheets of vital GC's, sandwiched between their basement membrane and vitelline membrane. Granulosa cell sheets of circa 4 mm2 were maintained in 35 mm culture dishes under serum-free conditions for up to 72 h in humidified room air at 37°C. The culture medium was M199 (Sigma, Bornem, Belgium) supplemented with 0.1% bovine serum albumin (fraction V, Sigma), 6.0 g/l HEPES, 50 U/ml penicillin and 50 mg/ml streptomycin at pH 7.4. The medium was changed every 24 h. In order to inhibit the apoptotic process culture medium was supplemented with 100 ng/ml sheep pituitary FSH (NIH-FSH-S17 U/mg Sigma) in control cultures.40
Enzyme histochemistry
Living GC sheets were stained at 37°C, pH 7.4 during 40 to 90 min with 2 mg/ml DAB (3-3′ diaminobenzidine-tetrahydrochloride dihydrate) that was freshly prepared in M199 culture medium according to a modification of the technique previously described.41 Oxidation of DAB is effected by cytochrome c which is itself reoxidized by cytochrome oxidase. The reaction thus requires both the presence of cytochrome c and cytochrome oxidase and will localize only the co-presence of both enzymes. An excess of catalase is added to the medium, in order to exclude reaction products caused by peroxidatic reactions. Controls were incubated in DAB-medium containing 10−3 M KCN as cytochrome oxidase inhibitor. After the incubation the unfixed sheets were fixed in a 4% formaldehyde solution buffered with Na-cacodylate containing 1% CaCl2; all the sheets were postosmicated for 1 h in 1% osmiumtetroxide in water and after dehydration embedded in epon. The same cytochrome c incubation procedure was performed on GC-sheets prefixed during 5 min in the above mentioned formaldehyde fixative. Ultrathin sections were contrasted with uranylacetate and lead and analysed on a Jeol electron microscope.
For light microscopial evaluation whole mounts of the sheets were counterstained with methylgreen.
Cytochrome oxidase assay
Cytochrome oxidase activity was measured spectrofotometrically according to a previously validated technique.42
Detection of cytochrome c release by Western blotting
GC sheets cultured for 72 h under serum-free conditions and uncultured (control) GC sheets were incubated in medium containing 0.2% collagenase and rotated for 10 min at 37°C. Cells were harvested by centrifugation and washed four times with cold PBS. For the detection of cytosolic cytochrome c, cells were lysed in lysis buffer containing 0.075% digitonin, 10 mM HEPES-NaOH pH 7.4, 220 mM mannitol, 68 mM sucrose, 2 mM NaCl, 2.5 mM KH2PO4, 0.5 mM EGTA, 2 mM MgCl2, 5 mM pyruvate, 0.1 mM PMSF and 1 mM dithiothreitol. Unsoluble material was pelleted by centrifugation and the supernatant was retained as a source for cytosolic cytochrome c. The cell pellet was further extracted with 10 mM Tris/HCl pH 7, 1% NP40, 200 mM NaCl, 5 mM EDTA and 1 mM PMSF to determine the mitochondrial cytochrome c. Proteins were subjected to SDS–PAGE and transferred to Hybond nitrocellulose filters (Amersham Pharmacia Biotech, Upssala, Sweden), blocked with 5% dry milk in PBS containing 0.3% Tween 20 and probed with anti-cytochrome c (clone 7H8.2C12, Pharmingen, San Diego, CA, USA) as described by the manufacturer's protocol. Blots were developed with the chemiluminescence method (NENTM Renaissance, NEN Life Science Products, Inc.).
Quantitation of apoptosis by DAPI staining
At the end of each culture period (24, 48, 72 h), GC-sheets were rinsed in PBS and fixed in 4% buffered formaldehyde. Then GC-sheets were washed again in PBS and stained with DAPI (4′,6′-diamidino-2-phenylindole; 1 mg/ml) in distilled water at room temperature for 10 min. Subsequently GC sheets were mounted on slides and samples were observed under an epifluorescence microscope (Leitz) using the DAPI bandpass. Apoptotic cells were identified as cells showing condensed chromatin or fragmented nuclei. A minimum of 1000 cells were scored for each condition and apoptosis was expressed as the per cent apoptotic nuclei. Nuclear fragments lying in close proximity were counted as one apoptotic nucleus.
Abbreviations
- AI:
-
apoptotic index
- DAB:
-
diaminobenzidine tetrahydrochloride dihydrate
- FSH:
-
follicle stimulating hormone
- GC:
-
granulosa cell
- zVAD-fmk:
-
Z-Val-Ala-Asp-fluoromethylketone
References
Kerr JF and Harmon BV . (1991) Definition and incidence of apoptosis: A historical perspective. In: Current Communications in Cell and Molecular Biology. Apoptosis: The molecular basis of cell death,Tomei LD and Cope FO, eds. vol. 3 (Cold Spring Harbor: Laboratory Press) pp5–29
Hockenbery D, Nunez G, Milliman C, Schreiber R and Korsmeyer S . (1990) Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348: 334–336
Newmeyer DD, Farschon DM and Reed JC . (1994) Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell 79: 353–364
Lazebnik YA, Cole S, Cooke CA, Nelson WG and Earnshaw WC . (1993) Nuclear events of apoptosis in vitro in cell-free mitotic extracts: A model system for analysis of the active phase of apoptosis. J. Cell Biol. 123: 7–22
Richter C, Schweizer M, Cossarizza A and Franceshi C . (1996) Control of apoptosis by cellular levels of ATP. FEBS Lett. 378: 107–110
Tsujimoto Y . (1997) Apoptosis and necrosis: Intracellular ATP level as a determinant for cell death modes. Cell Death Differ. 4: 429–434
Nicotera P and Leist M . (1997) Energy supply and the shape of death in neurons and lymphoid cells. Cell Death Differ. 4: 435–442
Kroemer G . (1997) Mitochondrial implication in apoptosis. Towards an endosymbiont hypothesis of apoptosis evolution. Cell Death Differ. 4: 443–456
Liu X, Kim CN, Yang J, Jemmerson R and Wang X . (1996) Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochromec. Cell 86: 147–157
Green DR and Reed JC . (1998) Mitochondria and apoptosis. Science 281: 1309–1312
Murphy AN . (1999) Potential mechanisms of mitochondrial cytochrome-c release during apoptosis. Drug Develop. Res. 46: 18–25
Kluck RM, Bossy-Wetzel E, Green DR and Newmeyer DD . (1997) The release of cytochromec from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 275: 1132–1136
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP and Wang X . (1997) Prevention of apoptosis by Bcl-2: release of cytochromec from mitochondria blocked. Science 275: 1129–1132
Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B and Borner C . (1998) Bcl-2 prolongs cell survival after Bax-induced release of cytochromec. Nature 391: 496–499
D'Herde K and Leybaert L . (1997) Intracellular free calcium related to apoptotic cell death in quail granulosa cell sheets kept in serum-free culture. Cell Death Differ. 4: 59–65
D'Herde K and Leybaert L . (1998) Apoptotic granulosa cells have moderately increased intracellular free calcium during phosphatidylserine exposure and a normal resting calcium level during DNA fragmentation. Apoptosis 3: 337–343
Zhuang J, Dinsdale D and Cohen GM . (1998) Apoptosis, in human monocytiic THP.1 cells, results in the release of cytochromec prior to their ultracondensation, formation of outer membrane discontinuities and reduction in inner membrane potential. Cell Death Differ. 5: 953–962
Orrenius S, Burgess DH, Hampton MB and Zhivotovsky B . (1997) Mitochondria as the focus of apoptosis research. Cell Death Differ. 4: 427–428
Reed JC . (1997) Cytochromec: Can't live with it–can't live without it. Cell 91: 559–562
Smiley ST, Reers M, Mottola-Harshorn C, Lin M, Chen A, Smith TW, Steele Jr GD and Chen LB . (1991) Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation. Proc. Natl. Acad. Sci. USA 88: 3671–3675
Camilleri-Bröet S, Vanderwerff H, Caldwell E and Hockenbery D . (1998) Distinct alterations in mitochondrial mass and function characterize different models of apoptosis. Exp. Cell Res. 239: 277–292
Bradham CA, Qian T, Streetz K, Trautwein C, Brenner DA, Lemasters JJ . (1998) The mitochondrial permeability transition is required for tumor necrosis factor alpha-mediated apoptosis and cytochromec release. Mol. Cell. Biol. 18: 6353–6364
Diaz G, Setzu MD, Zucca A, Isola R, Diana A, Murru R, Sogos V and Gremo F . (1999) Subcellular heterogeneity of mitochondrial membrane potential: relationship with organelle distribution and intercellular contacts in normal, hypoxic and apoptotic cells. J. Cell Sci. 112: 1077–1084
Li F, Srinivasan A, Wang Y, Armstrong RC, Tomaselli KJ and Fritz LC . (1997) Cell-specific induction of apoptosis by microinjection. J. Biol. Chem. 272: 30299–30305
Zhivotovsky B, Orrenius S, Brustugun OT and Doskeland SO . (1998) Injected cytochromec induces apoptosis. Nature 391: 449–450
Bossy-Wetzel E, Newmeyer D and Green D . (1998) Mitochondrial cytochromec release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 17: 37–49
Neame SJ, Rubin LL and Philpott KL . (1998) Blocking cytochromec activity within intact neurons inhibits apoptosis. J. Cell Biol. 142: 1583–1593
Andreyev AY, Fahy B and Fiskum G . (1998) Cytochromec release from brain mitochondria is independent of the mitochondrial permeability transition. FEBS Lett. 439: 373–376
Manaon S, Chaudhuri B and Guérin M . (1997) Release of cytochromec and decrease of cytochromec oxidase in Bax-expressing yeast cells, and prevention of these effects by coexpression of Bcl-XL. FEBS Lett. 415: 29–32
Krippner A, Matsumo-Yagi A, Gottlieb RA and Babior BM . (1996) Loss of function of cytochromec in jurkat cells undergoing fas-mediated apoptosis. J. Biol. Chem. 271: 21629–21636
Angermüller S, Künstle G and Tiegs G . (1998) Pre-apoptotic alterations in hepatocytes of TNF-a treated galactosamine-sensitized mice. J. Histochem. Cytochem. 46: 1175–1183
Mancini M, Anderson BO, Caldwell E, Sedginasab M, Paty PB and Hochenbery DM . (1997) Mitochondrial proliferation and paradoxical membrane depolarization during terminal differentiation and apoptosis in a human colon carcinoma cell line. J. Cell Biol. 138: 449–469
Hackenbrock CR . (1968) Ultrastructural bases for metabolically linked mechanical activity in mitochondria. II. Electron transport-linked ultrastructural transformation in mitochondria. J. Cell Biol. 37: 345–369
Hackenbrock CR, Rehn TG, Weinbach EC and Lemasters JJ . (1971) Oxidative phosphorylation and ultrastructural transformation in mitochondria in the intact ascites tumor cell. J. Cell Biol. 51: 123–131
Martinou I, Desagher S, Eskes R, Antonsson B, André E, Fakan S and Matinou JC . (1999) The release of cytochromec from mitochondria during apoptosis of NGF-deprived sympathetic neurons in a reversible event. J. Cell Biol. 144: 883–889
Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT and Thompson CB . (1997) Bcl-Xl regulates the membrane potential and volume homeostasis of mitochondria. Cell 91: 627–637
Borner C and Monney L . (1999) Apoptosis without caspases: an inefficient molecular guillotine? Cell Death Differ. 6: 497–507
Schotte P, Declercq W, Van Huffel S, Vandenabeele P and Beyaert R . (1999) Non-specific effects of methyl ketone peptide inhibitors of Caspases. FEBS Lett. 442: 117–121
Gilbert AB, Evans AJ, Perry MM and Davidson MH . (1977) A method for separating the granulosa cells, the basal lamina and the theca of the preovulatory ovarian follicle of the domestic fowl (Gallus domesticusf). J. Reprod. Fertil. 50: 179–181
Tilly JL and Tilly KI . (1995) Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinol. 136: 242–252
Roels F . (1974) Cytochromec and cytochrome oxidase in diaminobenzidine staining of mitochondria. J. Histochem. Cytochem. 22: 442–444
DiMauro S, Servidei S, Zeviani M, Di Rocco M, De Vivo DE, Didonato S, Uziel G, Berry K, Hoganson G and Johnsen SD . (1987) Cytochromec oxidase deficiency in Leigh syndrome. Ann. Neurol. 22: 498–506
Seligman AM, Karnovsky MJ, Wasserkrug HL and Hanker JS . (1968) Non droplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilicreagent, diaminobenzidine (DAB). J. Cell Biol. 38: 1–13
Acknowledgements
The authors are indebted to N Verweire and D Jacobus for excellent technical assistance. This study was supported by the BOF (Bijzonder Onderzoeksfonds) to K D'Herde 01115099. P Schotte and R Beyaert are researchers with the IWT and FWO-Vlaanderen, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by JC Reed
Rights and permissions
About this article
Cite this article
D'Herde, K., De Prest, B., Mussche, S. et al. Ultrastructural localization of cytochrome c in apoptosis demonstrates mitochondrial heterogeneity. Cell Death Differ 7, 331–337 (2000). https://doi.org/10.1038/sj.cdd.4400655
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4400655
Keywords
This article is cited by
-
Phosphorylation disrupts long-distance electron transport in cytochrome c
Nature Communications (2022)
-
The virulent Wolbachia strain wMelPop increases the frequency of apoptosis in the female germline cells of Drosophila melanogaster
BMC Microbiology (2012)
-
Life and death of female gametes during oogenesis and folliculogenesis
Apoptosis (2008)
-
Gap junctions and the propagation of cell survival and cell death signals
Apoptosis (2005)
-
Proteins That Fuse and Fragment Mitochondria in Apoptosis: Con-Fissing a Deadly Con-Fusion?
Journal of Bioenergetics and Biomembranes (2005)