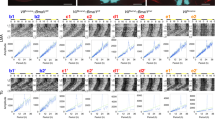
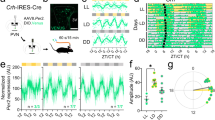
Abstract
Mammals manifest circadian behaviour timed by an endogenous clock in the hypothalamic suprachiasmatic nucleus (SCN)1. Considerable progress has been made in identifying the molecular basis of the circadian clock2,3, but the mechanisms by which it is translated into cyclic firing activity, high during the day and low at night, are still poorly understood. GABA (γ-aminobutyric acid), a common inhibitory neurotransmitter in the central nervous system, is particularly densely distributed within the SCN, where it is located in the majority of neuronal somata4,5 and synaptic terminals6,7. Using an in vitro brain-slice technique, we have now studied the effect of bath-applied GABA on adult SCN neurons at various times of the day. We find that GABA acts as an inhibitory neurotransmitter at night, decreasing the firing frequency; but during the day GABA acts as an excitatory neurotransmitter, increasing the firing frequency. We show that this dual effect, which is mediated by GABAA receptors, may be attributed to an oscillation in intracellular chloride concentration. A likely explanation is that the amplitude of the oscillation in firing rate, displayed by individual neurons, is amplified by the dual effect of GABA in the SCN's GABAergic network.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Klein, D. C., Moore, R. Y. & Reppert, S. M. (eds) Suprachiasmatic Nucleus: The Mind's Clock (Oxford University Press, New York, (1991)).
Myers, M. P. et al. Positional cloning and sequence analysis fo the Drosophila clock gene, timeless. Science 270, 805–808 (1995).
Page, T. L. Time is the essence: Molecular analysis of the biological clock. Science 263, 1570–1572 (1994).
Moore, R. Y. & Speh, J. C. GABA is the principal neurotransmitter of the circadian system. Neurosci. Lett. 150, 112–116 (1993).
Buijs, R. M. et al. Ultrastructural evidence for intra- and extranuclear projections of GABAergic neurons of the suprachiasmatic nucleus. J. Comp. Neurol. 340, 381–391 (1994).
Castel, M. et al. GABAergic innervation of the mouse suprachiasmatic nucleus. Eur. J. Neurosci.(suppl.) 3, Abstr. 2, 111 (1990).
Decavel, C. & van den Pol, A. N. GABA: A dominant neurotransmitter in the hypothalamus. J. Comp. Neurol. 302, 1019–1037 (1990).
Okamura, H. et al. Demonstration of GABAergic cell bodies in the suprachiasmatic nucleus: In situ hybridization of glutamic acid decarboxylase (GAD) mRNA and immunocytochemistry of GAD and GABA. Neurosci. Lett. 102, 131–136 (1989).
Buijs, R. M. et al. Colocalization of γ-aminobutyric acid with vasopressin, vasoactive intenstinal peptide, and somatostatin in the rat supachiasmatic nucleus. J. Comp. Neurol. 358, 343–352 (1995).
Belenky, M. et al. The suprachiasmatic nucleus in stationary organotypic culture. Neuroscience 70, 127–143 (1996).
Strecker, G. J. & Dudek, F. E. Local synaptic circuits in the rat suprachiasmatic nucleus. Soc. Neurosci.(Abstr.) 20, 1439 (1994).
Strecker, G. J. et al. Neurotransmission and electrophysiological mechanisms in the suprachiasmatic nucleus. Sem. Neurosci. 7, 43–51 (1995).
Welsh, D. K. et al. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14, 697–706 (1995).
Ralph, M. R. & Menaker, M. GABA regulation of circadian responses to light. I. Involvement of GABAA-benzodiazepine and GABAB receptors. J. Neurosci. 9, 2858–2865 (1989).
Smith, R. D. et al. Bicuculline and picrotoxin block phase advances induced by GABA agonists in the circadian rhythm of locomotor activity in the golden hamster by a phaclofen-insensitive mechanism. Brain Res. 530, 275–282 (1990).
Collinge, J. et al. Prion protein is necessary for normal synaptic function. Nature 370, 295–297 (1994).
Tobler, I. et al. Altered circadian activity and sleep in mice devoid of prion protein. Nature 380, 639–642 (1996).
Estibeiro, J. P. Multiple roles for PrP in the prion diseases. Trends Neurosci. 19, 257–258 (1996).
Prosser, R. A. & Gillette, M. U. The mammalian ciradian clock in the suprachiasmatic nucleus is reset in vitro by cAMP. J. Neurosci. 9, 1073–1081 (1989).
Gillette, M. U. in Suprachiasmatic Nucleus: The Mind's Clock (eds Klein, D. C., Moore, R. Y. & Reppert, S. M.) 125–143 (Oxford University Press, New York, (1991)).
Mason, R. et al. The effects of GABA and benzodiazepines on neurones in the suprachiasmatic nucleus (SCN) of Syrian hamsters. Brain Res. 552, 53–57 (1991).
Bos, N. P. A. & Mirmiran, M. Effects of excitatory and inhibitory amino acids on neuronal discharges in the cultured suprachiasmatic nucleus. Brain Res. Bull. 31, 67–72 (1993).
Liou, S. Y. & Albers, H. E. Single unit response of neurons within the hamster suprachiasmatic nucleus to GABA and low chloride perfusate during the day and night. Brain Res. Bull. 25, 93–98 (1990).
Staley, K. J. et al. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science 269, 977–980 (1995).
Cherubini, E. et al. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 14, 515–519 (1991).
Obrietan, K. & van den Pol, A. N. GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. J. Neurosci. 15, 5065–5077 (1995).
Andersen, P. et al. Two different responses of hippocampal pyramidal cells to application of γ-aminobutyric acid. J. Physiol. (Lond.) 305, 279–296 (1980).
Castel, M. et al. Glutamate-like immunoreactivity in retinal terminals of the mouse suprachiasmatic nucleus. Eur. J. Neurosci. 5, 368–381 (1993).
Acknowledgements
We thank M. Belenky for perfecting the organotypic slice explant culture technique; H. Matzner and S. Cohen for technical assistance; and J. Morris for critical reading of the text. Research was supported by the USA–Israel Binational Science Foundation and the Israel Ministry of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wagner, S., Castel, M., Gainer, H. et al. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature 387, 598–603 (1997). https://doi.org/10.1038/42468
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/42468
This article is cited by
-
Intracellular chloride regulation mediates local sleep pressure in the cortex
Nature Neuroscience (2023)
-
Daily rhythm in cortical chloride homeostasis underpins functional changes in visual cortex excitability
Nature Communications (2023)
-
Potential of Capric Acid in Neurological Disorders: An Overview
Neurochemical Research (2023)
-
Systematic review of drugs that modify the circadian system’s phase-shifting responses to light exposure
Neuropsychopharmacology (2022)
-
Diurnal rhythm regulates the frequency of carbachol-induced beta oscillation via inhibitory neural system in rat hippocampus
Cognitive Neurodynamics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.