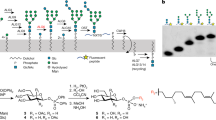
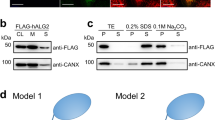
Abstract
N-linked glycosylation of proteins in eukaryotic cells follows a highly conserved pathway. The tetradecasaccharide substrate (Glc3Man9GlcNAc2) is first assembled at the membrane of the endoplasmic reticulum (ER) as a dolichylpyrophosphate (Dol-PP)-linked intermediate, and then transferred to nascent polypeptide chains in the lumen of the ER1. The assembly of the oligosaccharide starts on the cytoplasmic side of the ER membrane with the synthesis of a Man5GlcNAc2-PP-Dol intermediate. This lipid-linked intermediate is then translocated across the membrane so that the oligosaccharides face the lumen of the ER, where the biosynthesis of Glc3Man9GlcNAc2-PP-Dol continues to completion. The fully assembled oligosaccharide is transferred to selected asparagine residues of target proteins. The transmembrane movement of lipid-linked Man5GlcNAc2 oligosaccharide is of fundamental importance in this biosynthetic pathway, and similar processes involving phospholipids and glycolipids are essential in all types of cells2,3,4. The process is predicted to be catalysed by proteins, termed flippases, which to date have remained elusive2,3,4. Here we provide evidence that yeast RFT1 encodes an evolutionarily conserved protein required for the translocation of Man5GlcNAc2-PP-Dol from the cytoplasmic to the lumenal leaflet of the ER membrane.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kornfeld, R. & Kornfeld, S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 54, 631–664 (1985).
Bugg, T. D. & Brandish, P. E. From peptidoglycan to glycoproteins: common features of lipid-linked oligosaccharide biosynthesis. FEMS Microbiol. Lett. 119, 255–262 (1994).
Higgins, C. F. Flip-flop: the transmembrane translocation of lipids. Cell 79, 393–395 (1994).
Sprong, H., van der Sluijs, P. & van Meer, G. How proteins move lipids and lipids move proteins. Nature Rev. Mol. Cell Biol. 2, 504–513 (2001).
Hettema, E. H. & Tabak, H. F. Transport of fatty acids and metabolites across the peroxisomal membrane. Biochim. Biophys. Acta 1486, 18–27 (2000).
Borst, P., Zelcer, N. & van Helvoort, A. ABC transporters in lipid transport. Biochim. Biophys. Acta 1486, 128–144 (2000).
Abeijon, C. & Hirschberg, C. B. Topography of glycosylation reactions in the endoplasmic reticulum. Trends. Biochem. Sci. 17, 32–36 (1992).
Burda, P. & Aebi, M. The dolichol pathway of N-glycosylation. Biochim. Biophys. Acta 1426, 239–257 (1999).
Perez, M. & Hirschberg, C. B. Topography of glycosylation reactions in the rough endoplasmic reticulum membrane. J. Biol. Chem. 261, 6822–6830 (1986).
Snider, M. D. & Rogers, O. C. Transmembrane movement of oligosaccharide-lipids during glycoprotein synthesis. Cell 36, 753–761 (1984).
McCloskey, M. A. & Troy, F. A. Paramagnetic isoprenoid carrier lipids. 1. Chemical synthesis and incorporation into model membranes. Biochemistry 19, 2056–2060 (1980).
Hanover, J. A. & Lennarz, W. J. The topological orientation of N,N′- diacetylchitobiosylpyrophosphoryldolichol in artificial and natural membranes. J. Biol. Chem. 254, 9237–9246 (1979).
Huffaker, T. C. & Robbins, P. W. Yeast mutants deficient in protein glycosylation. Proc. Natl. Acad. Sci. USA 80, 7466–7470 (1983).
Cipollo, J. F., Trimble, R. B., Chi, J. H., Yan, Q. & Dean, N. The Yeast ALG11 gene specifies addition of the terminal alpha 1,2-Man to the Man5GlcNAc2-PP-dolichol N-glycosylation intermediate formed on the cytosolic side of the endoplasmic reticulum. J. Biol. Chem. 276, 21828–21840 (2001).
Ng, D. T., Spear, E. D. & Walter, P. The unfolded protein response regulates multiple aspects of secretory and membrane protein biogenesis and endoplasmic reticulum quality control. J. Cell Biol. 150, 77–88 (2000).
Davidson, E. A. & Gowda, D. C. Glycobiology of Plasmodium falciparum. Biochimie 83, 601–604 (2001).
Hasilik, A. & Tanner, W. Carbohydrate moiety of carboxypeptidase Y and perturbation of its biosynthesis. Eur. J. Biochem. 91, 567–575 (1978).
Aebi, M., Gassenhuber, J., Domdey, H. & te Heesen, S. Cloning and characterization of the ALG3 gene of Saccharomyces cerevisiae. Glycobiology 6, 439–444 (1996).
Orlean, P. Dolichol phosphate mannose synthase is required in vivo for glycosyl phosphatidylinositol membrane anchoring, O-mannosylation, and N-glycosylation of protein in Saccharomyces cerevisiae. Mol. Cell Biol. 10, 5796–5805 (1990).
Orlean, P., Kuranda, M. J. & Albright, C. F. Analysis of glycoproteins from Saccharomyces cerevisiae. Methods Enzymol. 194, 682–697 (1991).
Jelinek-Kelly, S. & Herscovics, A. Glycoprotein biosynthesis in Saccharomyces cerevisiae. Purification of the alpha-mannosidase which removes one specific mannose residue from Man9GlcNAc. J. Biol. Chem. 263, 14757–14763 (1988).
Rush, J. S. & Wächter, C. J. Transmembrane movement of a water-soluble analogue of mannosylphosphoryldolichol is mediated by an endoplasmic reticulum protein. J. Cell Biol. 130, 529–536 (1995).
Zhou, Q. et al. Molecular cloning of human plasma membrane phospholipid scramblase. A protein mediating transbilayer movement of plasma membrane phospholipids. J. Biol. Chem. 272, 18240–18244 (1997).
Schaffer, J. E. & Lodish, H. F. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell 79, 427–436 (1994).
Feldman, M. F. et al. The activity of a putative polyisoprenol-linked sugar translocase (Wzx) involved in Escherichia coli O antigen assembly is independent of the chemical structure of the O repeat. J. Biol. Chem. 274, 35129–35138 (1999).
Guthrie, C. & Fink, G. R. Guide to yeast genetics and molecular biology. Methods Enzymol. 194, 3–37 (1991).
Zufferey, R. et al. STT3, a highly conserved protein required for yeast oligosaccharyltransferase activity in vitro. EMBO J. 14, 4949–4960 (1995).
Wach, A. et al. in Methods in Microbiology: Yeast Gene Analysis (eds Tuite, M. & Brown, A. J.) 67–81 (Academic, San Diego, 1998).
Acknowledgements
We thank C. J. Waechter for his contributions. We also thank F. Reggiori and A. Conzelmann, and T. Immervoll and M. Gentzsch for providing antibodies against Gas1 protein and chitinase, respectively. P. Orlean provided the dpm1-6 strain. This work was supported by grants from the Swiss National Science Foundation to M.A., the National Institutes of Health to P.W. and D.T.W.N., and the Canadian Institutes of Health Research to M.A.V. P.W. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Helenius, J., Ng, D., Marolda, C. et al. Translocation of lipid-linked oligosaccharides across the ER membrane requires Rft1 protein. Nature 415, 447–450 (2002). https://doi.org/10.1038/415447a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/415447a
This article is cited by
-
Injection of a Self-propelled Polymer into a Small Circular Cavity
Chinese Journal of Polymer Science (2024)
-
Translocation of a Self-propelled Polymer through a Narrow Pore
Chinese Journal of Polymer Science (2022)
-
Complexity of the eukaryotic dolichol-linked oligosaccharide scramblase suggested by activity correlation profiling mass spectrometry
Scientific Reports (2021)
-
Simulation study for the pulling translocation of a polymer globule
Polymer Journal (2021)
-
Visualizing conformation transitions of the Lipid II flippase MurJ
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.