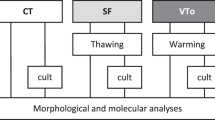
Frozen banking of whole organs for transplantation is starting to look feasible.
Abstract
Transplantation surgery, which is limited by the supply and short-term viability of fresh donor organs, would be revolutionized if these could survive freezing, but early claims of cryopreservation1 were never realized. Here we describe the successful transplantation in rats of ovaries, fallopian tubes and the upper segment of the uterus en bloc after storage in liquid nitrogen.
Similar content being viewed by others
Main
We anaesthetized adult female Lewis rats using sodium pentobarbital and removed the right ovary and the reproductive tract, with the ovarian vessels dissected to create short cuffs of aorta and vena cava2,3; these preparations were held in solution for less than an hour before transplantation. Syngeneic recipients were anaesthetized, heparinized and oophorectomized. The aorta and vena cava below the left renal artery were dissected for end-to-side aortic–aorta and veno–venous anastomoses using continuous suturing; the uterus was joined to the corresponding host segment. After paired operations lasting for 2 h, circulation was restored to donor organs (for further details, see supplementary information). All surviving recipients stayed healthy.
We used eight fresh organs for transplantation and also seven treated ones that we had perfused for 30 min at 0.35 ml min−1 with M2 medium containing 0.1 M fructose and increasing concentrations of dimethylsulphoxide (0–1.5 M). Following a protocol previously used for sliced ovarian tissue4, we cooled the treated organs slowly in 5-ml cryovials (Nalgene), inducing ice nucleation at −7 °C in the modified chamber of an automated freezer (CryoLogic). After overnight storage in liquid nitrogen, we rapidly thawed the vials and removed the cryoprotectant by perfusion with a reversed concentration gradient (for further details, see supplementary information).
We monitored ovarian function by taking vaginal smears (see supplementary information) and by pairing some animals with stud males four weeks later. Ten weeks after surgery, we collected blood immediately after euthanasia to measure serum concentrations of follicle-stimulating hormone (FSH) and oestradiol-17β by radioimmunoassay and chemiluminescence, respectively. We prepared organs for histology and to assess ovarian follicle reserves.
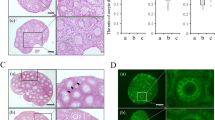
All animals with fresh transplants reinitiated oestrus. They had normal numbers of ovarian follicles, including graafian stages (Fig. 1a), and uterine weights and FSH levels were similar to those of controls (9.7 ± 0.5 and 9.1 ± 0.4 ng ml−1, respectively). Four animals with cryopreserved grafts (57%) had follicular ovaries, and their corpora lutea indicated recent ovulation (Fig. 1b). One animal was pregnant, with two healthy fetuses implanted on either side of the uterine anastomosis (Fig. 1c). This group had higher serum FSH levels (21.3 ± 6.2 ng ml−1), fewer follicles and lower oestradiol levels and uterine weights, but none was in the castrate range. Tubal and uterine morphology were indistinguishable from unoperated controls.
a, Fresh-ovary transplants had growing follicles (arrow) and mature graafian follicles (GF). b, Cryopreserved-ovary transplants were also follicular and contained corpora lutea (CL). c, Two fetuses conceived by one animal that had received a frozen–thawed transplant. Scale bars: 0.05 mm (a, b) and 5 mm (c).
These results are encouraging, but indicate that ovarian function is compromised to some extent by freezing, perhaps because of intravascular ice formation — a problem that has previously frustrated attempts to cryopreserve kidneys5. Advances in vitrification may overcome this problem if the chemical toxicity of additives can be minimized6. Frozen banking of reproductive organs could eventually be useful in breeding from endangered species and as a fertility option for women and children who have undergone sterilizing chemotherapy7,8. Our success with ovarian transplants should stimulate investigation into the improvement of cryopreservation techniques for other organs.
References
Guttman, F. M. et al. Cryobiology 14, 559–567 (1977).
Lee, S. J. Res. Inst. Sci. Korea 6, 112 (1974).
Cornier, E., Sibella, P. & Chatelet, F. J. Gynécol. Obstet. Biol. Reprod. 14, 567–573 (1985).
Gosden, R. G. et al. Hum. Reprod. 9, 597–603 (1994).
Jacobsen, I. A. et al. Cryobiology 21, 637–653 (1984).
Song, Y. C. et al. Nature Biotechnol. 18, 296–299 (2000).
Picton, H. M., Kim, S. S. & Gosden, R. G. Br. Med. Bull. 56, 603–615 (2000).
Radford, J. A. et al. Lancet 357, 1172–1175 (2001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing financial interests: declared none.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, X., Chen, H., Yin, H. et al. Fertility after intact ovary transplantation. Nature 415, 385 (2002). https://doi.org/10.1038/415385a
Issue Date:
DOI: https://doi.org/10.1038/415385a
This article is cited by
-
Biobanked human foreskin epithelial cell sheets reduce inflammation and promote wound healing in a nude mouse model
BMC Biotechnology (2021)
-
New method of FACS analyzing and sorting of intact whole ovarian fragments (COPAS) after long time (24 h) cooling to 5 °C before cryopreservation
Cell and Tissue Banking (2021)
-
Successful fertility following optimized perfusion and cryopreservation of whole ovary and allotransplantation in a premature ovarian insufficiency rat model
Journal of Ovarian Research (2018)
-
Fertility preservation through gonadal cryopreservation
Reproductive Medicine and Biology (2016)
-
Advances in cryopreservation of organs
Journal of Huazhong University of Science and Technology [Medical Sciences] (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.