Abstract
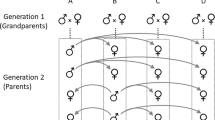
Individual males in many animal species exhibit discrete modes of behaviour1,2,3, but the genetic mechanisms underlying these differences are poorly understood. Here we investigate the genetics of the isopod crustacean Paracerceis sculpta, in which three different types of males coexist, each distinguishable from the others by their behavioural and morphological phenotypes4,5. Within families, alleles of the gene encoding the enzyme phosphoglucomutase (Pgm gene) are associated with particular male phenotypes, although no significant association between these characters exists population-wide. This suggests that Pgm is closely linked to a single genetic locus which controls male phenotype. We call this the alternative mating strategy (Ams) locus. We present evidence that two other factors—an autosomal gene, transformer (Tfr), and an extrachromosomal factor—interact with primary sex determination loci and with alleles at Ams, causing certain individuals to change sex, thereby biasing family sex ratios. A model based on our genetic analysis suggests that: first, polymorphism in male behaviour is controlled by the mendelian segregation of three alleles at the Ams locus; second, that family sex ratio is influenced by alternative alleles at the Tfr locus whose expression is influenced by the extrachromosomal factor; and third, that Tfr and Ams interact epistatically to determine the sex of the individual and, if male, its behaviour and external morphology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lank, D. B., Smith, C. M., Hanotte, O., Burke, T. & Cooke, F. Genetic polymorphism for alternative mating behaviour in lekking male ruff. Nature 378, 59–62 (1995).
Gross, M. R. Alternative reproductive strategies and tactics; diversity within sexes. Trends Ecol. Evol. 11, 92–97 (1996).
Sinervo, B. & Lively, C. M. The rock–paper–scissors game and the evolution of alternative male strategies. Nature 380, 240–243 (1996).
Shuster, S. M. Alternative reproductive behaviors: Three discrete male morphs in Paracerceis sculpta, an intertidal isopod from the northern Gulf of California. J. Crust. Biol. 7, 318–327 (1987).
Shuster, S. M. The reproductive behaviour of α-, β-, and γ-males in Paracerceis sculpta, a marine isopod crustacean. Behaviour 121, 231–258 (1992).
Shuster, S. M. Male alternative reproductive behaviors in a marine isopod crustacean (Paracerceis sculpta): The use of genetic markers to measure differences in fertilization success among α-, β- and γ-males. Evolution 34, 1683–1698 (1989).
Shuster, S. M. & Wade, M. J. Equal mating success among male reproductive strategies in a marine isopod. Nature 350, 606–61 (1991).
Sassaman, C. Inbreeding and sex ratio variation in female-biased populations of a clam shrimp, Eulimnadia texana. Bull. Mar. Sci. 45, 425–432 (1989).
Hartl, R. & Clark, A. in Principles of Population Genetics2nd edn, 47–57 (Sinauer, Sunderland, MA, (1989)).
Heath, D. J. & Ratford, J. R. The inheritance of sex ratio in the isopod, Sphaeroma rugicauda. Heredity 64, 419–425 (1990).
Bull, J. J. Evolution of Sex Determining Mechanisms(Benjamin-Cummings, Menlo Park, CA, (1983)).
Legrand, J. J., Legrand-Hamelin, E. & Juchault, P. Sex determination in Crustacea. Biol. Rev. 62, 439–470 (1987).
Juchault, P., Rigaud, T. & Mocquard, J. -P. Evolution of sex-determining mechanisms in a wild population of Armadillidium vulgare Latr. (Crustacea: Isopoda): competition between two feminizing parasitic sex factors. Heredity 69, 382–390 (1992).
Rousset, F., Bouchon, D., Pintureau, B., Juchault, P. & Solignac, M. Wolbachia endosymbionts responsible for various alterations of sexuality in Arthropods. Proc. R. Soc. Lond. B 250, 91–98 (1991).
Hurst, L. D. The incidences, mechanisms and evolution of cytoplasmic sex ratio distorters in animals. Biol. Rev. 68, 121–193 (1993).
Rigaud, T. & Juchault, P. Conflict between feminizing sex ratio distorters and an autosomal masculinizing gene in the terrestrial isopod, Armadillidium vulgareLatr. Genetics 133, 247–252 (1993).
Juchault, P. & Rigaud, T. Evidence for female heterogamety in two terrestrial crustaceans and the problem of sex chromosome evolution in isopods. Heredity 75, 488–471 (1995).
Read, T. R. C. & Cressie, N. A. C. in Goodness-of-fit Statistics for Discrete Multivariate Data 136–139 (Springer, New York, (1988)).
Shuster, S. M. & Wade, M. J. Female copying and sexual selection in a marine isopod crustacean. Anim. Behav. 42, 1071–1078 (1991).
Shuster, S. M. in Crustacean Sexual Biology(eds Bauer, R. T. & Martin, J. W.) 91–110 (Columbia Univ. Press, New York, (1991)).
Levins, R. Evolution in Changing Environments(Princeton Univ. Press, Princeton, (1968)).
Slatkin, M. On the equilibration of fitnesses by natural selection. Am. Nat. 112, 845–859 (1978).
Maynard Smith, J. Evolution and the Theory of Games(Cambridge Univ. Press, New York, (1982)).
Lively, C. M. Canalization versus developmental conversion in a spatially variable environment. Am. Nat. 128, 561–572 (1986).
Slatkin, M. The evolutionary response to frequency and density dependent interactions. Am. Nat. 114, 384–398 (1979).
Wright, S. Evolution and Genetics of Populations Vol. 2(Univ. of Chicago Press(1969)).
Wade, M. J., Shuster, S. M. & Stevens, L. Bottlenecks, founder events and inbreeding: Experimental studies of the response to selection with Tribolium. Evolution 50, 723–733 (1996).
Shuster, S. M. Changes in female anatomy associated with the reproductive molt in Paracerceis sculpta (Holmes), a semelparous isopod crustacean. J. Zool. 225, 365–379 (1991).
Shuster, S. M. Courtship and female mate selection in a semelparous isopod crustacean (Paracerceis sculpta). Anim. Behav. 40, 390–399 (1990).
Tinturier-Hamelin, E. Sur le polychromatisme de l'isopode Flabellifere Dynamene bidentata (Adams) II. Etude genetique du mutant bimaculata partiellement. Cah. Biol. Mar. 4, 473–591 (1967).
Acknowledgements
This research was supported by the NSF and by organized research and departmental funding from Northern Arizona University, and was authorized by the Mexican Government. We thank M. Wade and B. Charlesworth for reviewing data and earlier drafts of the manuscript; Y. Toquenaga for statistical advice and for a program for calculating exact χ2 tests; D. Dorado, S. Juarez, S. Hag, H. Baitoo, S. Bhakta, Y. Bhakta, S. Brekhus; H. Yoon; N. Kim, M. Kim, U. Rao and L. Lynch for assistance in maintaining laboratory animals; and V. Jormalainen, P. Nelson, K.Johnson, G. Davis and M. Pitts for discussion.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shuster, S., Sassaman, C. Genetic interaction between male mating strategy and sex ratio in a marine isopod. Nature 388, 373–377 (1997). https://doi.org/10.1038/41089
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/41089
This article is cited by
-
Sexual selection on population-level mating opportunities drives morph ratios in a fig wasp with extreme male dimorphism
BMC Ecology and Evolution (2021)
-
Dimorphic male squid show differential gonadal and ejaculate expenditure
Hydrobiologia (2018)
-
Genetic mapping of the female mimic morph locus in the ruff
BMC Genetics (2013)
-
Variation in developmental arrest among male orangutans: a comparison between a Sumatran and a Bornean population
Frontiers in Zoology (2013)
-
Spiders do not escape reproductive manipulations by Wolbachia
BMC Evolutionary Biology (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.