Abstract
The API2-MALT1 fusion gene was originally identified from a t(11;18)(q21;q21) translocation, a specific chromosomal abnormality that is found in mucosa-associated lymphoid tissue (MALT) lymphoma. Gastric MALT lymphomas positive for the API2-MALT1 fusion gene do not respond to Helicobacter pylori–eradication therapy, but otherwise, the incidence and clinicopathological behavior of colorectal MALT lymphoma with this genetic abnormality are unclear. We examined the API2-MALT1 fusion by multiplex RT-PCR method in 47 cases of MALT lymphoma and 13 cases of diffuse large B-cell lymphoma and evaluated the relevance of API2-MALT1 positivity to the clinical and pathological features. The mean ages of MALT lymphoma and diffuse large B-cell lymphoma patients were 65 (range, 37–87 y) and 58 (range, 14–85 y) years, respectively. API2-MALT1 fusion genes were detected in seven cases (15%) of MALT lymphoma and one case (8%) of diffuse large B-cell lymphoma. In MALT lymphomas, the tumor size in API2-MALT1–positive cases was 62 ± 39 mm (mean ± SD), statistically larger than that in API2-MALT1–negative cases (25 ± 19 mm; P < .01). The API2-MALT1–positive cases demonstrated more advanced clinical stages and a male predominance, compared with API2-MALT1–negative cases. Thus, API2-MALT1–positive tumors should be cared for as a more aggressive subgroup and be followed for a longer time.
Similar content being viewed by others
Main
Mucosa-associated lymphoid tissue (MALT) lymphomas are unique tumors that originate from acquired MALT associated with chronic inflammation or autoimmune responses, e.g., Helicobacter pylori–associated chronic gastritis, Hashimoto’s thyroiditis, Sjögren’s syndrome, etc. (1, 2). MALT lymphoma affects various organs, among which the stomach is the most frequently involved (1, 2, 3). Interestingly, the majority of gastric low-grade MALT lymphoma cases, those proven to be neoplastic by detection of monoclonal rearrangement in immunoglobulin (Ig) genes, regress after eradication of H. pylori (4, 5).
Recently, the t(11;18) chromosomal translocation was identified as a specific chromosomal abnormality in some MALT lymphomas (6, 7, 8), and the API2-MALT1 fusion gene was found to be associated with this abnormality (9, 10, 11). API2 contains three sets of baculovirus inhibitor of apoptosis repeats (BIRs), a caspase recruitment domain (CARD), and a RING finger domain. API2 is thought to suppress apoptosis by inhibiting caspases and activation of NF-κB (12, 13). MALT1 contains Ig-like domains that bind BCL10 (9, 11, 14). Gastric MALT lymphomas with this abnormality do not respond to H. pylori eradication (15, 16, 17, 18, 19). Therefore, two types of gastric MALT lymphomas may exist: one associated with H. pylori–induced gastritis and negative for t(11;18) and the other positive for t(11;18) and histogenetically not directly related to H. pylori infection.
MALT lymphoma is less frequently found in the large intestine (3, 20, 21), and in this site often exhibits a submucosal tumor, macroscopically. Only a few reports have described the regression of colorectal MALT lymphomas to antibiotic treatments that are generally found to be successful for gastric tumors (22, 23). The presence of the t(11;18) translocation in colorectal MALT lymphomas has occasionally been reported (24, 25, 26), but, to our knowledge, the incidence and clinicopathological characteristics of lymphomas with, compared with without, this abnormality have not yet been addressed.
MATERIALS AND METHODS
Cases
Formalin-fixed, paraffin-embedded tissues from 60 cases of MALT lymphoma and diffuse large B-cell lymphoma of the colorectum were retrieved from the pathology files at the Okayama University Graduate School of Medicine and Dentistry, Aichi Cancer Center Hospital, Kawasaki Medical School Hospital, and Wakayama Medical University between 1989 and 2000. All cases were reviewed to confirm the histological diagnosis according to the criteria of the World Health Organization Classification for Tumors of Hematopoietic and Lymphoid Tissues (1). We diagnosed colorectal MALT lymphomas by the findings as follows: centrocyte-like cells and/or monocytoid cells proliferation, especially at the outside of the mantle zone (marginal zone distribution); presence of plasmacytic differentiation and/or follicular colonization in some cases; and absence of CD5, CD10, and cyclin D1 overexpression. Lymphoepithelial lesions in colorectal MALT lymphoma were not as obvious as those of gastric tumors were (representative histologies were shown in Fig. 1). All patients were Japanese. Lymphomas that involved the colon and rectum secondarily were not included in the study. Clinical data for each case were obtained from pathological reports and medical reports. As summarized in Table 1, the number of MALT lymphomas was 47 (17 from males and 30 from females), and that of diffuse large B-cell lymphoma was 13 (7 from males and 6 from females). Mean ages of MALT lymphoma and diffuse large B-cell lymphoma cases were 65 (range, 37–87 y) and 58 (range, 14–85 y) years, respectively. A sample from each case was used for molecular analysis and immunohistochemistry.
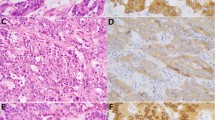
Histology of MALT lymphoma and diffuse large B-cell lymphoma of the colon and rectum. A and B, histology of the API2-MALT1–positive MALT lymphoma of the colon. C, histology of the API2-MALT1–negative MALT lymphoma of the colon. D, plasmacytoid differentiation of the API2-MALT1–negative MALT lymphoma (arrowhead, Dutcher body). E and F, histology of the API2-MALT1–positive diffuse large B-cell lymphoma with low-grade MALT lymphoma component (Case 8 in Fig. 3). E is large-cell component and F is low-grade component. Note numerous mitotic figures in E (arrowheads) that are hardly seen in F.
Immunohistochemistry
To confirm the diagnosis of MALT lymphoma and diffuse large B-cell lymphoma, immunohistochemistry was performed using the indirect immunoperoxidase method with secondary antibodies conjugated to peroxidase-labeled dextran polymer (EnVision+; DAKO Japan, Kyoto, Japan), according to the manufacturer’s instructions. The primary monoclonal antibodies (MAbs) were against the following antigens: CD20 and CD79a, purchased from DAKO Japan; CD5 and CD10, from Novocastra Lab (Newcastle, U.K.); and cyclin D1, from ZYMED (San Francisco, CA). The MALT lymphoma cells were positive for CD20 and CD79a and negative for CD5, CD10, and cyclin D1.
Immunostaining for the BCL10 protein was also performed. In brief, 3-μm-thick tissue sections were heat retrieved in 10 mm citric acid buffer (pH 6.0) in a microwave oven for 30 minutes. These were incubated with an anti-BCL10 antibody, diluted 1:100 (ZYMED), at 4° C overnight, and were subjected to the indirect immunoperoxidase method using EnVision+. Finally, they were visualized by 3′3-diaminobenzidine HCl in H2O2. A nuclear staining pattern of >10% of the lymphoma cells was defined as positive for BCL10 and was independently determined by two pathologists to make a consensus evaluation.
Detection of Clonality
DNA was extracted from unstained, formalin-fixed, paraffin-embedded samples. Clonality was examined as described previously (27, 28). In brief, amplification of IgH genes was performed by semi-nested PCR, using primers directed to the Framework 2 or Framework 3 region and to the joining region. At least two DNA samples were extracted from each paraffin block and separately subjected to PCR reaction. The DNA amplification of each material was carried out more than once. The amplified products from each patient were electrophoresed in parallel. The determination as “clonal” was made only when a single or dominant discrete band was consistently reproduced from different specimens.
Detection of the API2-MALT1 Fusion Gene
AP12-MALT1 fusion genes were detected using the multiplex RT-PCR method as described previously (29). Briefly, 3-mm-thick, deparaffinized sections were incubated in digestion buffer (20 mm Tris, pH 8.0, 20 mm EDTA, 1% SDS, and 1 mg/mL proteinase K) at 56° C overnight. Total RNA was extracted using TRIZOL LS (Invitrogen Japan, Tokyo, Japan). RT-PCR primers are listed in Table 2. Reverse transcription was performed using SuperscriptII (Invitrogen Japan) at 42° C for 30 minutes. The PCR program was as follows; 40 cycles at 95° C for 30 seconds, at 50° C for 45 seconds, and at 72° C for 1 minute for the first round; and 35 cycles at 95° C for 15 seconds, at 55° C for 30 seconds, and at 72° C for 45 seconds for the second round. The amplified products were separated on a 4% agarose gel and purified. Purified samples were directly sequenced by Big Dye terminators (Applied Biosystems Japan, Tokyo, Japan) and analyzed on an ABI prism 310 genetic analyzer (Applied Biosystems).
Statistical Analysis
Student’s t test and Fisher’s exact probability test were used to compare clinical data or BCL 10 immunostaining positivity between the API2-MALT1–positive and –negative cases.
RESULTS
Clinical Features of MALT Lymphoma and Diffuse Large B-Cell Lymphoma
In a total of 60 patients, the median follow-up periods ranged from 1 to 133 months, except for 5 patients who failed to be followed. With regard to clinical stages, 61% of the patients with MALT lymphoma and 30% of the patients with diffuse large B-cell lymphoma remained at Stage I. In both types of lymphoma, 20% of patients advanced to Stage IV. Lymph nodal involvement was seen in 13 cases; 9 cases: regional lymph nodes, 2: paraaortic lymph nodes, 1: paravaginal lymph nodes, and 1: systemic lymph nodes. Extranodal involvement was seen as follows; 3 cases in the stomach, 3 in the small intestine, and 1 in the thyroid, bone marrow, and lungs, respectively. In all cases, the main organ was colorectum, and clinical records showed colorectal origin. Cases that were thought to be secondary involvement or primary organ undetermined were not included in the present series.
According to International Prognostic Index, the risk was found to be low in 71% of patients with MALT lymphoma and in 63% of patients with diffuse large B-cell lymphoma. Approximately one half of the patients received only resection (surgical or endoscopic) therapy. A combined therapy of resection and chemotherapy was performed on 13 patients. Eradication of H. pylori was used in 8 patients, but a therapeutic effect was not clear in all of these patients. Relapse of lymphoma occurred in 6% of MALT lymphoma cases and in 15% of diffuse large B-cell lymphoma cases. Most patients with MALT lymphoma were alive during the follow-up period. The patients with diffuse large B-cell lymphoma also demonstrated a respectively good survival rate (80%). See Tables 1 and 3 for further specifics on patient clinical features.
Pathological Features of MALT Lymphoma and Diffuse Large B-Cell Lymphoma of the Colon and Rectum
The most frequent location of tumors in the 60 lymphomas was the rectum (29 cases, 48%). In MALT lymphomas, 27 cases arose in the rectum, followed by 7 cases in multiple sites (5 cases involving the rectum) and 5 cases in the cecum. Diffuse large B-cell lymphoma cases did not seem to have such a predilection, with cases almost equally involving the cecum, ascending colon, rectum, and multiple sites.
Elevated lesions (polyp or submucosal tumor) were formed in 89% of MALT lymphoma cases and 31% of diffuse large B-cell lymphoma. Ulcerative lesions were formed much more frequently in diffuse large B-cell lymphoma (62%) than MALT lymphomas (6%).
The average sizes of MALT lymphomas and diffuse large B-cell lymphomas were 31 mm and 62 mm, respectively. About half of the MALT lymphomas were localized in the submucosa, whereas 80% of the diffuse large B-cell lymphoma cases involved the subserosa or deeper tissues. Histologically, plasmacytic differentiation was found in seven MALT lymphomas often associated with Dutcher bodies (Fig. 1). Three of 13 cases of diffuse large B-cell lymphoma included low-grade MALT lymphoma components (Fig. 1).
Clinical Outcome and Therapeutic Ways
Clinical outcome, therapeutic methods, and responses were summarized in Table 3. In 35 MALT lymphoma cases of Clinical Stage I or II, 29 cases (83%) achieved complete remission, and 6 (17%) were partial response or refractory. In contrast, in nine Stage IV cases, two (22%) were complete remission, and seven (78%) were partial response or refractory. In eight cases of diffuse large B-cell lymphoma at Clinical Stage I or II, seven cases were complete remission and one case was partial response or refractory. In two diffuse large B-cell lymphoma cases at Clinical Stage IV, one achieved complete remission, and the other case was partial response or refractory.
There were various kinds of therapeutic ways, and we summarized them into two groups: one group with surgical or endoscopic resection (resected group) and another group without resections (unresected group). In MALT lymphoma cases, 39 were in the resected group and 5 were in the unresected group. In the resected group, 30 cases achieved complete remission, and 9 cases were partial response or refractory. In the unresected group, 1 was complete remission, and 4 were partial response or refractory. For diffuse large B-cell lymphomas, seven were in the resected group, and all achieved complete remission. The other three cases were in the unresected group, and one was complete remission and two were partial response or refractory.
In the present study, four patients examined died: two MALT lymphoma cases and two diffuse large B-cell lymphomas; one was at Stage I, another one at Stage II, and the other two at Stage IV. The MALT lymphoma patient at clinical Stage I died of urothelial carcinoma, and the other three cases died of lymphoma.
Clonalities and Detection of API2-MALT1 Fusion Gene
As shown in Table 1, monoclonality was detected in 73% of MALT lymphomas and 83% of diffuse large B-cell lymphomas.
API2-MALT1 fusion genes were detected in 7 cases (15%) of MALT lymphoma and 1 case (8%) of diffuse large B-cell lymphoma (Fig. 2, Table 1). This diffuse large B-cell lymphoma contained a MALT lymphoma component (Fig. 1). All cases had the A1446 breakpoint in the API2 gene on the 11th chromosome. As for the MALT1 gene on 18th chromosome, 5 cases had the M814 breakpoint, 1 had the M1123 breakpoint, and 2 had the M1150 breakpoint (Fig. 3).
RT-PCR amplification for the API2-MALT1 fusion gene and for β-actin gene isolated from the lymphomas. Lanes 1–7, the API2-MALT1–positive cases of MALT lymphoma; Lane 8, the API2-MALT1–positive case of diffuse large B-cell lymphoma with MALT lymphoma component; Lane 9, a representative case of MALT lymphoma without API2-MALT1 fusion gene; N1 and N2, negative control (N1, reactive lymph node hyperplasia; N2, RNA-free sample); M, molecular markers. The numbers of Lanes 1–8 correspond to the case numbers in Figure 3, respectively.
Structure of detected API2-MALT1 fusion genes. All cases have the A1446 breakpoint in the API2 gene. As for the MALT1 gene, Cases 1, 2, 3, 5, and 7 have the M814 breakpoint; Case 6 has the M1123 breakpoint; and Cases 4 and 8 have the M1150 breakpoint. Numbering is according to API2 accession number L49432 and MALT1 accession number AF130356.
Relevance of API2-MALT1 Positivity to the Clinical and Pathological Features
In MALT lymphomas, the mean tumor size of API2-MALT1–positive cases was 62 ± 38 mm (mean ± SD), statistically larger than that of API2-MALT1–negative cases (25 ± 19 mm; P < .01). The clinical stage and patient gender were also statistically different between API2-MALT1–positive and negative patients (P < .001 and P < .01, respectively). The age, depth of tumors, number of tumors, shape, and International Prognostic Index demonstrated no significant relevance to API2-MALT1 positivity (Table 4).
Correlation of BCL 10 Nuclear Expression and API2-MALT1 Fusion Gene
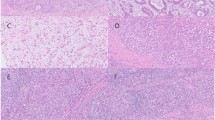
BCL10 immunostaining was performed and evaluated in 30 cases of MALT lymphoma and 10 of diffuse large B-cell lymphoma. The positive nuclear immunostaining pattern for BCL 10 was observed in 9 cases of MALT lymphoma and 4 cases of diffuse large B-cell lymphoma. Nuclear immunostaining of BCL10 was displayed at a significantly higher rate in the API2-MALT1–positive MALT lymphomas (P < .01; Table 4). In the API2-MALT1–positive diffuse large B-cell lymphoma, the positive nuclear pattern for BCL 10 was detected in the proliferating large cells (Fig. 4).
BCL 10 immunostaining of MALT lymphoma and diffuse large B-cell lymphoma of the colon and rectum. A and B, BCL 10 immunostaining in API2-MALT1–positive diffuse large B-cell lymphoma with MALT lymphoma component (Case 8). A, nuclear and cytoplasmic expression of BCL10 in large lymphoma cells. Note BCL10–negative nuclei of intermingling cells (arrowheads). B, nuclear and cytoplasmic expression of BCL10 in MALT lymphoma component. C, BCL 10 immunostaining in API2-MALT1–negative MALT lymphoma. Only cytoplasmic reactivity is seen without nuclear-expression pattern.
DISCUSSION
We examined clinicopathological features of 47 MALT lymphomas and 13 diffuse large B-cell lymphoma arising from the colon and rectum. The patients with MALT lymphoma negative for API2-MALT1 fusion gene exhibited a female predominance (occurrence approximately three times more commonly than in males), but diffuse large B-cell lymphoma cases did not show such a tendency. This might be related to the fact that MALT lymphomas are often associated with an autoimmune state (1, 30), which is known to occur more frequently in females. In contrast, the API2-MALT1–positive cases actually demonstrated a male predominance in our series. As the number of API2-MALT1–positive cases was rather small, it may be difficult to form a conclusion. However, our data suggest that the lymphomagenesis of API2-MALT1–positive cases is different from that of other ordinary MALT lymphomas.
A few cases exhibiting regression of colorectal MALT lymphomas after eradication of H. pylori have been reported (22, 23). However, these cases may be etiologically related to unknown microorganisms, because H. pylori was not found in the colon or rectum. In our series, eight cases treated with antibiotics did not show any clear improvement. H. pylori was detected in five of eight cases that were treated with eradication. There was no clear relationship between H. pylori infection and t(11;18). In most cases, tumor cell infiltrates were mainly confined to the submucosa, forming elevated lesions (polypoid lesions or submucosal tumors) as previously reported (31). We did not observe significant differences in tumor shapes between API2-MALT1 positive and negative cases as reported in the gastric case (16). As tumors from API2-MALT1–positive cases often also displayed similar reactive germinal centers as those from API2-MALT1–negative cases (data not shown), chronic inflammation may be associated with lymphomagenesis even in API2-MALT1–negative cases. In contrast, most diffuse large B-cell lymphoma formed ulcerative lesions (similar to those in advanced cancers), were larger in diameter, and infiltrated more deeply than MALT lymphomas.
There was a good correlation between API2-MALT1 positivity and clinical stages in MALT lymphomas. API2-MALT1–positive cases had larger tumors, in more advanced clinical stages. Advanced tumor stage at diagnosis (including regional lymph node infiltration) is one of the predictive prognostic factors of gastrointestinal lymphomas (31, 32, 33, 34, 35). In our cases, however, there was no significant difference in prognosis between API2-MALT1 –positive and negative cases of MALT lymphoma. In MALT lymphoma cases, 42 patients were alive for the follow-up period, and only 1 patient died of lymphoma; the patient in the other case died of urothelial carcinoma. It is said that MALT lymphomas demonstrate indolent progression and result in a good prognosis. For patients diagnosed in the early stage of the gastric lymphoma, the 5-year survival rate is >90%, and 10-year survival is about 70% (32, 34). Our data indicate that colorectal lymphomas also have a good prognosis as a whole. However, it has been reported that MALT lymphomas in the advanced stage are resistant to chemotherapy and have a rather poor prognosis (34, 35). Therefore, the API2-MALT1–positive group might have a poor prognosis if longer follow-up data are examined. As shown in Table 3, achievement of complete remission was closely related to lower clinical stage. Most patients were treated with surgery or endoscopic resection, and being in the unresected group may result in failure of remission, though such cases were rather small in number. Patient death seemed to relate to advanced clinical stage.
BCL 10 is a novel gene cloned from the t(11;18) translocation breakpoint, and its product functions as one of the apoptosis regulatory molecules (36, 37). In the present study, most cases positive for the API2- MALT1 fusion gene displayed nuclear immunostaining with BCL10, which is compatible with a previous report (38). BCL10 binds to the Ig-like domains of MALT1, and the complex functions in the inhibition of apoptosis (39). What kind of other abnormalities involved in t(11;18)–negative cases that had nuclear expression of BCL10 remains unclear now. The API2-MALT1 fusion gene was detected in one of the 13 diffuse large B-cell lymphoma cases. In this case, which contained a low-grade MALT lymphoma component, nuclear staining of BCL10 was observed in large lymphoma cells as well as MALT lymphoma cells. This is supportive evidence for high-grade progression of the low-grade component. We checked BCL-6 expression and found that large cells of this case expressed BCL-6 (data not shown). This finding is similar to that in a previous report of gastric lymphoma (40). Some allelic imbalances and microsatellite instability were found in both t(11;18)–negative MALT lymphomas and diffuse large B-cell lymphoma, but rarely in t(11;18)–positive MALT lymphomas (41, 42). This is compatible with previous reports that t(11;18) is not detectable in diffuse large B-cell lymphoma (43, 44, 45, 46). However, our findings indicate the possibility that t(11;18)–positive MALT lymphoma could, on some occasions, transform into diffuse large B-cell lymphoma when an additional genetic aberration was added. Interestingly, we also have a similar case in ocular adnexa lymphomas that has recently been published in this journal(47).
In summary, most colorectal MALT lymphomas demonstrate indolent progression, and therefore, partial resection is thought to be the first line of therapy. API2-MALT1–positive tumors tend to be larger in size and in the advanced stage. These should be cared for as a more aggressive subgroup and be followed for a longer time.
References
Isaacson PG, Berger F, Müller-Hermelink HK, Nathwani BN, Piris MA, Swerdlow SH, et al. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Tumours of haematopoietic and lymphoid tissues. World Health Organization classification of tumors, pathology and genetics. Lyon, France: IARC Press; 2001. p. 157–160.
Isaacson PG . Mucosa-associated lymphoid tissue lymphoma. Semin Hematol 1999; 36: 139–147.
Isaacson PG . Gastrointestinal lymphomas of T- and B-cell types. Mod Pathol 1999; 12: 151–158.
Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993; 342: 575–577.
Thiede C, Wündisch T, Neubauer B, Alpen B, Morgner A, Ritter M, et al. Eradication of Helicobacter pylori and stability of remissions in low-grade gastric B-cell lymphomas of the mucosa-associated lymphoid tissue: results of an ongoing multicenter trial. Recent Results Cancer Res 2000; 156: 125–133.
Auer IA, Gascoyne RD, Connors JM, Cotter FE, Greiner TC, Sanger WG, et al. t(11;18)(q21;q21) is the most common translocation in MALT lymphomas. Ann Oncol 1997; 8: 979–985.
Ott G, Katzenberger T, Greiner A, Kalla J, Rosenwald A, Heinrich U, et al. The t(11;18)(q21;q21) chromosome translocation is a frequent and specific aberration in low-grade but not high-grade malignant non-Hodgkin’s lymphomas of the mucosa-associated lymphoid tissue (MALT-) type. Cancer Res 1997; 57: 3944–3948.
Dierlamm J, Wlodarska I, Michaux L, Stefanova M, Hinz K, Van Den Berghe H, et al. Genetic abnormalities in marginal zone B-cell lymphoma. Hematol Oncol 2000; 18: 1–13.
Dierlamm J, Baens M, Wlodarska I, Stefanova-Ouzounova M, Hernandez JM, Hossfeld DK, et al. The apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11;18)(q21;q21) associated with mucosa-associated lymphoid tissue lymphomas. Blood 1999; 93: 3601–3609.
Akagi T, Motegi M, Tamura A, Suzuki R, Hosokawa Y, Suzuki H, et al. A novel gene, MALT1 at 18q21, is involved in t(11;18)(q21;q21) found in low-grade B-cell lymphoma of mucosa-associated lymphoid tissue. Oncogene 1999; 18: 5785–5794.
Morgan JA, Yin Y, Borowsky AD, Kuo F, Nourmand N, Koontz JI, et al. Breakpoints of the t(11;18)(q21;q21) in mucosa-associated lymphoid tissue (MALT) lymphoma lie within or near the previously undescribed gene MALT1 in chromosome 18. Cancer Res 1999; 59: 6205–6213.
Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV . The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 1995; 83: 1243–1252.
LaCasse EC, Baird S, Korneluk RG, MacKenzie AE . The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene 1998; 17: 3247–3259.
Lucas PC, Yonezumi M, Inohara N, McAllister-Lucas LM, Abazeed ME, Chen FF, et al. Bcl10 and MALT1, independent targets of chromosomal translocation in MALT lymphoma, cooperate in a novel NF-κB signaling pathway. J Biol Chem 2001; 276: 19012–19019.
Alpen B, Neubauer A, Dierlamm J, Marynen P, Thiede C, Bayerdörfer E, et al. Translocation t(11;18) absent in early gastric marginal zone B-cell lymphoma of MALT type responding to eradication of Helicobacter pylori infection. Blood 2000; 95: 4014–4015.
Nakamura T, Nakamura S, Yonezumi M, Suzuki T, Matsuura A, Yatabe Y, et al. Helicobacter pylori and the t(11;18)(q21;q21) translocation in gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type. Jpn J Cancer Res 2000; 91: 301–309.
Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, Ye H, Molina T, Bouhnik Y, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet 2001; 357: 39–40.
Sugiyama T, Asaka M, Nakamura T, Nakamura S, Yonezumi S, Seto M . API2-MALT1 chimeric transcript is a predictive marker for the responsiveness of H. pylori eradication treatment in low-grade gastric MALT lymphoma. Gastroenterology 2001; 120: 1884–1885.
Liu H, Ye H, Ruskone-Fourmestraux A, De Jong D, Pileri S, Thiede C, et al. t(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology 2002; 122: 1286–1294.
Morton JE, Leyland MJ, Vaughan Hudson G, Vaughan Hudson B, Anderson L, Bennett MH, et al. Primary gastrointestinal non-Hodgkin’s lymphoma: a review of 175 British National Lymphoma Investigation cases. Br J Cancer 1993; 67: 776–782.
Koch P, del Valle F, Berdel WE, Willich NA, Reers B, Hiddemann W, et al. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001; 19: 3861–3873.
Raderer M, Pfeffel F, Pohl G, Mannhalter C, Valencak J, Chott A . Regression of colonic low grade B cell lymphoma of the mucosa associated lymphoid tissue type after eradication of Helicobacter pylori. Gut 2000; 46: 133–135.
Matsumoto T, Iida M, Shimizu M . Regression of mucosa-associated lymphoid-tissue lymphoma of rectum after eradication of Helicobacter pylori. Lancet 1997; 350: 115–116.
Motegi M, Yonezumi M, Suzuki H, Suzuki R, Hosokawa Y, Hosaka S, et al. API2-MALT1 chimeric transcripts involved in mucosa-associated lymphoid tissue type lymphoma predict heterogeneous products. Am J Pathol 2000; 156: 807–812.
Remstein ED, James CD, Kurtin PJ . Incidence and subtype specificity of API2-MALT1 fusion translocations in extranodal, nodal, and splenic marginal zone lymphomas. Am J Pathol 2000; 156: 1183–1188.
Yonezumi M, Suzuki R, Suzuki H, Yoshino T, Oshima K, Hosokawa Y, et al. Detection of AP12-MALT1 chimaeric gene in extranodal and nodal marginal zone B-cell lymphoma by reverse transcription polymerase chain reaction (PCR) and genomic long and accurate PCR analyses. Br J Haematol 2001; 115: 588–594.
Mannami T, Yoshino T, Oshima K, Takase S, Kondo E, Ohara N, et al. Clinical, histopathological, and immunogenetic analysis of ocular adnexal lymphoproliferative disorders: characterization of MALT lymphoma and reactive lymphoid hyperplasia. Mod Pathol 2001; 14: 641–649.
Yoshino T, Ichimura K, Mannami T, Takase S, Ohara N, Okada H, et al. Multiple organ mucosa-associated lymphoid tissue lymphomas often involve the intestine. Cancer 2001; 91: 346–353.
Inagaki H, Okabe M, Seto M, Nakamura S, Ueda R, Eimoto T . API2-MALT1 fusion transcripts involved in mucosa-associated lymphoid tissue lymphoma: multiplex RT-PCR detection using formalin-fixed paraffin-embedded specimens. Am J Pathol 2001; 158: 699–706.
Mackay IR, Rose NR . Autoimmunity and lymphoma: tribulations of B cells. Nat Immunol 2001; 2: 793–795.
Shepherd NA, Hall PA, Coates PJ, Levison DA . Primary malignant lymphoma of the colon and rectum. A histopathological and immunohistochemical analysis of 45 cases with clinicopathological correlations. Histopathology 1988; 12: 235–252.
Cogliatti SB, Schmid U, Schumacher U, Eckert F, Hansmann ML, Hedderich J, et al. Primary B-cell gastric lymphoma: a clinicopathological study of 145 patients. Gastroenterology 1991; 101: 1159–1170.
Krugmann J, Dirnhofer S, Gschwendtner A, Berresheim U, Greil R, Krugmann K, et al. Primary gastrointestinal B-cell lymphoma. A clinicopathological and immunohistochemical study of 61 cases with an evaluation of prognostic parameters. Pathol Res Pract 2001; 197: 385–393.
Montalbán C, Castrillo JM, Abraira V, Serrano M, Bellas C, Piris MA, et al. Gastric B-cell mucosa-associated lymphoid tissue (MALT) lymphoma. Clinicopathological study and evaluation of the prognostic factors in 143 patients. Ann Oncol 1995; 6: 355–362.
Fisher RI, Dahlberg S, Nathwani BN, Banks PM, Miller TP, Grogan TM . A clinical analysis of two indolent lymphoma entities: mantle cell lymphoma and marginal zone lymphoma (including the mucosa-associated lymphoid tissue and monocytoid B-cell subcategories): a Southwest Oncology Group study. Blood 1995; 85: 1075–1082.
Willis TG, Jadayel DM, Du MQ, Peng H, Perry AR, Abdul-Rauf M, et al. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell 1999; 96: 35–45.
Zhang Q, Siebert R, Yan M, Hinzmann B, Cui X, Xue L, et al. Inactivating mutations and overexpression of BCL10, a caspase recruitment domain-containing gene, in MALT lymphoma with t(1;14)(p22;q32). Nat Genet 1999; 22: 63–68.
Liu H, Ye H, Dogan A, Ranaldi R, Hamoudi RA, Bearzi I, et al. t(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood 2001; 98: 1182–1187.
Yoneda T, Imaizumi K, Maeda M, Yui D, Manabe T, Katayama T, et al. Regulatory mechanisms of TRAF2-mediated signal transduction by Bcl10, a MALTlymphoma-associated protein. J Biol Chem 2000; 275: 11114–11120.
Omonishi K, Yoshino T, Sakuma I, Kobayashi K, Moriyama M, Akagi T . bcl-6 protein is identified in high-grade but not low-grade mucosa-associated lymphoid tissue lymphomas of the stomach. Mod Pathol 1998; 11: 181–185
Starostik P, Greiner A, Schultz A, Zettl A, Peters K, Rosenwald A, et al. Genetic aberrations common in gastric high-grade large B-cell lymphoma. Blood 2000; 95: 1180–1187.
Starostik P, Patzner J, Greiner A, Schwarz S, Kalla J, Ott G, et al. Gastric marginal zone B-cell lymphomas of MALT type develop along 2 distinct pathogenetic pathways. Blood 2002; 99: 3–9.
Rosenwald A, Ott G, Stilgenbauer S, Kalla J, Bredt M, Katzenberger T, et al. Exclusive detection of the t(11;18)(q21;q21) in extranodal marginal zone B cell lymphomas (MZBL) of MALT type in contrast to other MZBL and extranodal large B cell lymphomas. Am J Pathol 1999; 155: 1817–1821.
Baens M, Maes B, Steyls A, Geboes K, Marynen P, De Wolf-Peeters C . The product of the t(11;18), an API2-MLT fusion, marks nearly half of gastric MALT type lymphomas without large cell proliferation. Am J Pathol 2000; 156: 1433–1439.
Maes B, Baens M, Marynen P, De Wolf-Peeters C . The product of the t(11;18), an API2-MLT fusion, is an almost exclusive finding in marginal zone cell lymphoma of extranodal MALT-type. Ann Oncol 2000; 11: 521–526.
Kalla J, Stilgenbauer S, Schaffner C, Wolf S, Ott G, Greiner A, et al. Heterogeneity of the API2-MALT1 gene rearrangement in MALT-type lymphoma. Leukemia 2000; 14: 1967–1974.
Takada S, Yoshino T, Taniwaki M, Nakamura N, Nakamine H, Oshima K, et al. Involvement of the chromosomal translocation t(11;18) in some mucosa-associated lymphoid tissue lymphomas and diffuse large B-Cell lymphomas of the ocular adnexa evidence from multiplex reverse transcriptase-polymerase chain reaction and fluorescence in situ hybridization on using formalin-fixed, paraffin-embedded specimens. Mod Pathol 2003; 5: 445–452.
Acknowledgements
The authors thank Dr. K. Hayashi, Dr. Y. Ishii, Dr. I. Ito, Dr. T. Kishimoto, Dr. K. Miyatani, Dr. K. Mizobuchi, Dr. I. Murakami, Dr. S. Nose, Dr. K. Ogino, Dr. K. Taguchi, Dr. S. Takada, Dr. I. Yamadori, and Dr. M. Yoshida for providing lymphoma samples and thank Ms. M. Okabe for expert technical assistance.
Supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakugawa, S., Yoshino, T., Nakamura, S. et al. API2-MALT1 Fusion Gene in Colorectal Lymphoma. Mod Pathol 16, 1232–1241 (2003). https://doi.org/10.1097/01.MP.0000097283.47637.58
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.MP.0000097283.47637.58
Keywords
This article is cited by
-
Mucosa-associated lymphoid tissue (MALT) variant of primary rectal lymphoma: a review of the English literature
International Journal of Colorectal Disease (2017)
-
Methylation and API2/MALT1 fusion in colorectal extranodal marginal zone lymphoma
Modern Pathology (2009)
-
A dual role for the API2 moiety in API2-MALT1-dependent NF-κB activation: heterotypic oligomerization and TRAF2 recruitment
Oncogene (2007)
-
Deviated VH4 immunoglobulin gene usage is found among thyroid mucosa-associated lymphoid tissue lymphomas, similar to the usage at other sites, but is not found in thyroid diffuse large B-cell lymphomas
Modern Pathology (2006)