Abstract
RCAS1, a novel tumor-associated antigen, is expressed in advanced human neoplasias including uterine and ovarian carcinomas. RCAS1 protein was indicated to induce cell cycle arrest and apoptosis of cultured human lymphoid and myeloid cell lines and normal lymphocytes. In the present study, we investigated the expression and prognostic value of RCAS1 in 58 patients with colorectal carcinomas. RCAS1 protein was detected by immunoperoxidase staining using a mouse monoclonal anti-RCAS1 antibody (22-1-1 antibody). Immunohistochemical examination showed expression of RCAS1 in 75% of colorectal carcinomas with lymph node metastases (n = 24), whereas it was present in only 41% of tumors without metastases (n = 34, P < .05). Patients with RCAS1-positive tumors showed a significantly poorer prognosis than those negative for RCAS1 (P < .05). Multivariate analysis using the Cox regression model indicated that RCAS1 positivity was an independent negative predictor for survival (P = .0300; risk ratio, 0.496). In addition, apoptotic cells of tumor-infiltrating lymphocytes were examined using nonradioactive in situ nick translation in paraffin-embedded sections. The proportion of apoptotic tumor-infiltrating lymphocytes was significantly higher in RCAS1-positive colorectal carcinomas (11.2 ± 1.0) than in RCAS1-negative tumors (7.9 ± 1.0, P < .05). Our results suggest that overexpression of RCAS1 may negatively affect the prognosis of human colorectal carcinomas and that RCAS1 may play a role in tumor immune privilege in vivo.
Similar content being viewed by others
INTRODUCTION
The tumor-associated antigen RCAS1 (receptor-binding cancer antigen expressed on SiSo cells) was originally identified by a monoclonal antibody, 22-1-1, that was generated from mice immunized with a human uterine cervical adenocarcinoma cell line (1). Frequent expression of RCAS1 was demonstrated in uterine and ovarian carcinomas, especially in invasive carcinomas, but not in normal uterine cervical or ovarian tissues (2). Thereafter, a cDNA encoding RCAS1 was isolated from the human uterine adenocarcinoma cell line, SiSo (3). RCAS1 protein has a molecular weight of ≈45 kDa and consists of 213 amino acids with a transmembrane region at the N-terminus and a coiled-coil structure at the C-terminus, indicative of a Type II membrane protein (3). In immunostaining using the GST (glutathione S-transferase) fusion protein and anti-GST-detecting antibody, CD3- and CD16-positive lymphocytes were brightly stained (3). This finding suggested the presence of an RCAS1 receptor molecule on CD3-positive T cells and natural killer (NK) cells. The addition of RCAS1 molecules to preactivated peripheral lymphocytes or human lymphoma cell lines that express RCAS1 receptors inhibited their proliferation in vitro and induced cell death, mainly by apoptosis (3).
It has recently been demonstrated that Fas ligand (FasL)-expressing tumor cells can cause apoptosis of Fas-bearing T cells and NK cells (4, 5, 6, 7, 8). Lymphocyte depletion by Fas/FasL interaction may allow tumors to escape from immunological surveillance. As discussed above, RCAS1 is also expressed on various tumor cells and is known to induce cell growth inhibition and apoptosis of lymphocytes in vitro (3). Thus, RCAS1 may play an important role in the escape of tumor cells from immunological surveillance. Expression of RCAS1 correlates with invasion or progression in several carcinomas (2, 9, 10). In addition, RCAS1 expression is associated with poor prognosis in uterine, lung, gallbladder, pancreas, and esophageal carcinoma (11, 12, 13, 14, 15). Although previous studies suggested that RCAS1 is also expressed in colorectal carcinoma cells, the relationship between RCAS1 expression and prognosis in colorectal carcinomas is not known at present. In this study, we focused on RCAS1 expression in colorectal carcinomas and its prognostic value in colorectal cancer patients. Furthermore, the relationship of RCAS1 expression and the frequency of apoptosis among tumor-infiltrating lymphocytes (TIL) in vivo was investigated.
MATERIALS AND METHODS
Tissue Sections
Studies were conducted using 58 surgically resected specimens of primary colorectal adenocarcinomas, representing specimens harvested from all patients who underwent curative colectomy in the Second Department of Surgery, Nagasaki University Hospital, Nagasaki, Japan, from 1991 to 1994. None of these patients had received chemo-, radio-, or immunotherapy before tissue collection. Clinical follow-up data were available for all patients for ≤5 years after the operation. The excised colons were cut and tumor tissues were processed for histopathological examination. The specimens were fixed in 10% neutralized formalin, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin (HE). Tumors were classified according to the Dukes' classification. The characteristics of patients and tumors are summarized in Table 1.
Immunohistochemical Detection of RCAS1 Protein and Analysis of TIL
Mouse monoclonal antibody 22-1-1 (IgM) specifically recognizes RCAS1 antigen (1). In the present study, culture supernatants of hybridoma 22-1-1 were used for immunohistochemistry at 5-fold dilution. Paraffin-embedded sections of surgically resected colorectal tumors were deparaffinized in xylene and rehydrated before analysis. Slides were immersed in 0.3% H2O2 in methanol to block endogenous peroxidase and then preincubated with 5% BSA/PBS for 1 hour to block nonspecific binding of antibodies. Sections were reacted overnight at 4° C with anti-RCAS1 (22-1-1) antibody. After triplicate washing with PBS for 15 minutes each, sections were incubated with horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgM (1:200; DAKO, Carpinteria, CA) for 1 hour and washed three times with BSA for 10 minutes. The site of HRP was visualized using 3,3′-diaminobenzidine and H2O2. As a control, a few sections were incubated with an irrelevant mouse IgM instead of the 22-1-1 hybridoma supernatants at 5-fold dilution. Tissue sections with <5% reactive tumor cells were considered negative, and those with >5% reactive cells were defined as positive. To avoid reading bias, slides were prepared and read in a double-blind fashion by two investigators. Lymphocytes infiltrating colorectal tumors were identified immunohistochemically using mouse anti-human T lymphocyte antibody (UCHL1), mouse anti-human B lymphocyte (L26), and mouse anti-human NK cell-like (NK1; all from Dako). Briefly, paraffin-embedded samples were cut into 4- to 5-μm-thick sections, which were placed onto silane-coated glass slides, deparaffinized, and rehydrated. Immunostaining was performed as described above, except that HRP-rabbit anti-(mouse IgG) was used with anti-T- or B-cell antibodies, and HRP-rabbit anti-(mouse IgM) with anti-NK1 antibody. As a negative control, normal mouse IgG was used at the same dilution.
In Situ Detection of DNA Strand Breaks
To identify nuclei with DNA strand breaks at a cellular level, in situ nick translation (ISNT) was performed (16, 17). Briefly, paraffin-embedded sections (4 μm) were cut onto silane-coated glass slides and deparaffinized in a routine manner. After washing with PBS, sections were treated with proteinase K (PK; Sigma Chemical Co., St. Louis, MO) in PBS (1 μg/mL, 37° C, 15 min). After several washings with PBS, they were immersed in 50 mm Tris/HCl (pH 7.5). The reaction of ISNT was conducted for 3 hours at 37° C in a medium containing 50 mm Tris/HCl (pH 7.5), 10 mm MgCl2, 0.1 mm dithiothreitol, 50 μg/mL BSA, 200 U/mL DNA polymerase I (Takara Co., Tokyo, Japan), and 20 μm each of dATP, dGTP, dCTP, and biotin-11-dUTP or TTP, as described previously (18). The reaction was terminated by washing with 50 mm Tris-HCl buffer, pH 7.4, for 15 minutes. After incubation with 5% BSA in PBS for 1 hour at room temperature, sections were reacted with HRP-labeled anti-biotin antibody (Vector Laboratories, Burlingame, CA) dissolved in 5% BSA in PBS for 3 hours at room temperature. The signals were detected by using 3, 3′-diaminobenzidine, H2O2, CoCl2, and NiSO4(NH4) 2SO4. The labeling index (LI) for ISNT was determined by counting ISNT-positive nuclei among 1000 TILs in 10 areas per specimen as described previously (19).
Statistical Analysis
Statistical analyses were performed using the StatView II Ver. 5.0 software (Abacus Concept Inc., CA). All data were expressed as mean ± SE. The χ2 test was used to examine the association between RCAS1 expression and various clinicopathological characteristics. Differences between groups in the number of apoptotic cells were examined for statistical significance using the Student's t test. Survival rate was calculated by the Kaplan-Meier method. Multivariate regression analysis was performed using Cox proportional hazard model to determine the clinical pathological factors that influenced prognosis. A P value of <.05 denoted the presence of a statistically significant difference.
RESULTS
Expression of RCAS1 Antigen in Colorectal Carcinomas
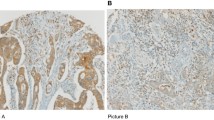
Expression of RCAS1 protein in colorectal cancer tissues was examined by immunohistochemistry using the anti-RCAS1 22-1-1 monoclonal antibody. Typical staining patterns of the tumors are shown in Figure 1. Staining for RCAS1 was detected both on the membrane and in the cytoplasm of colorectal cancer cells, but rarely in normal glands. The 22-1-1 antibody strongly stained deeply advanced areas of the tumor compared with adjacent glandular areas.
Immunohistochemical staining using anti-RCAS1 mouse monoclonal antibody (22-1-1) in paraffin-embedded colorectal carcinoma sections. A, RCAS1 was detected in colorectal carcinoma cells, but scarcely in normal mucosal glands. B, A representative tumor with RCAS1 expression on the cellular membrane and in the cytoplasm of deeply invasive colorectal carcinoma cells.
Clinical Significance of RCAS1 Expression in Colorectal Cancers
Of the 58 patients, 32 (55%) exhibited the expression of RCAS1 in tumors, whereas the remaining 26 cases did not show such expression. The reactive tumor cells ranged 5 to 80% (mean; 39.1 ± 6.8%, median; 37.5%) in cases with positive immunostaining (>5%). Table 1 summarizes the relationship between RCAS1 expression in colorectal tumors and various clinicopathological features. There was no significant relationship between RCAS1 expression and age, sex, tumor size, location, serum CEA titers, and histopathological classification. However, the percentage of cases positive for RCAS1 significantly increased with Dukes' stage (P < .01). Furthermore, the percentage of cases positive for RCAS1 was significantly higher in the presence of lymph node metastases (Dukes' C) than that without such metastasis (Dukes' A or B, P < .05). In addition, the percentage of cases positive for RCAS1 was significantly higher in Dukes' B carcinomas than in Dukes' A carcinomas in patients without lymph node metastases (P < .05).
Kaplan-Meier survival curves demonstrated that the 5-year survival rate in surgically treated patients who were positive for RCAS1 was 52.6%, which was markedly lower than 83.9% in those without such expression. Furthermore, there was a significant difference in the survival curves between patients with positive and negative RCAS1 expression (P < .05, log-rank test; Fig. 2). Although lymph node metastasis and LI of TIL (19) were independent prognostic factors in addition to RCAS1 expression, other variables were not independent predictors of prognosis.
Multivariate Survival Analysis of RCAS1 Expression and Clinical Factors
RCAS1 expression and clinicopathological factors for survival time were analyzed using the Cox proportional hazards model. The results indicated that lymph node metastasis (Dukes' A or B versus Dukes' C; P = .0079), LI of TIL (P = .0028) and RCAS1 positivity (P = .0300) were independent unfavorable prognostic factors in 58 patients of colorectal carcinomas (Table 2).
Cell Composition of TIL and Relationship between RCAS1 Expression and Apoptosis of TIL
Using serial sections, apoptosis of TIL in colorectal carcinomas was investigated by ISNT. Tumor cells were surrounded by TIL, which were predominantly composed of T cells (data not shown). Intense ISNT signals were detected in cells with morphological features of apoptosis (Fig. 3A). Control sections incubated with ISNT solutions without biotinylated dUTP were not stained (data not shown). The LI of apoptotic cells in ISNT sections was determined by counting ISNT-positive nuclei among 1000 TIL nuclei that were in the periphery of the tumors. The LI of apoptotic TIL ranged from 2 to 23 (mean, 9.7 ± 0.7 for all tumors). There were no significant differences in the mean LI values for TIL based on age, sex, tumor size, location, and histopathological type of colorectal carcinomas. On the other hand, the LI of TIL in RCAS1-expressing colorectal tumors was significantly higher (11.2 ± 1.0, n = 32) than in those negative for RCAS1 (7.9 ± 1.0, n = 26, P < .05; Fig. 3B).
ISNT staining of a representative colorectal carcinoma positive for RCAS1. A,ISNT-identified positive nuclei of TIL in the border zone between the tumor and normal colonic glands (arrows). B, the labeling index of patients with RCAS1 expression was significantly higher than that of patients without RCAS1 expression.
DISCUSSION
RCAS1 is widely expressed in various cancer tissues including uterine cervical carcinoma, ovarian carcinoma (1, 2), esophageal carcinomas, pancreatic carcinomas, gastric carcinomas, and colorectal carcinomas (2). Although RCAS1 gene is ubiquitously expressed in various organs such as the ovary, testis, prostate, thymus, muscle, and heart, as demonstrated in Northern blots, RCAS1 protein is not detected by immunohistochemistry in normal tissues including the above organs as well as peripheral blood lymphocytes, lymph nodes, small intestine, and colon (3). Furthermore, recent studies suggested that RCAS1 is not detected in uterine cervical dysplasias but only in a small proportion of carcinoma in situ or microinvasive uterine carcinoma (11). Our results were in agreement with those of the above studies; staining for RCAS1 was only positive in colorectal cancer cells but was scarcely detected in adenomas and normal glands. The 22-1-1 antibody strongly stained the deeply invasive part of colorectal carcinomas. These findings confirmed that RCAS1 is a tumor-associated antigen. In addition, we focused the relationship between RCAS1 expression and frequency of apoptotic change among TIL in colorectal carcinoma patients. The major findings in the present study were positive correlation of RCAS1 expression and the number of apoptotic TIL in patients of colorectal carcinoma.
In the present study, we investigated the expression of RCAS1 in 58 cases of colorectal carcinomas who were treated by surgical resection of the tumor and assessed it for the prognostic value of the expression. Immunohistochemical studies revealed that RCAS1 was detected in 75% (18 of 24 total cases) of colorectal carcinomas with lymph node metastases. RCAS1 protein was expressed in 55% (12 of 22 total cases) of Dukes' B colorectal carcinomas in contrast to 17% of Dukes' A carcinoma (2 of 12 total cases) in cases without lymph node metastases. In addition to a significant difference in the survival rate that was noted between patients who were positive for RCAS1 expression and those without such expression, multivariate analysis indicated that RCAS1 positivity is an independent prognostic factor in colorectal carcinoma patients. Thus, our results indicate that RCAS1 is preferentially expressed in the colorectal carcinomas with lymph node metastases or with invasion beyond the muscularis propria but rarely in early stage carcinomas or adenomas (not shown) and that RCAS1 expression is a clinically significant variable for estimating the prognosis of patients with colorectal cancers.
Interaction between tumor and host cells plays an important role in tumor progression (20). It has been demonstrated that the number of TIL around a tumor directly correlates with prognosis in colorectal carcinoma (21, 22). Apoptosis of TIL may cause depletion of lymphocytes and negatively affects prognosis. Apoptotic signals directed against lymphocytes have been studied extensively in Fas-FasL system (23, 24, 25). FasL is expressed in the stroma of the eye and Sertoli cells of the testis and is known to induce apoptosis of infiltrating lymphocytes (5, 8). As a consequence, these sites acquire immune privilege that facilitates allogenic or xenogeneic tissue grafts. Tumor escape from immunological surveillance via the Fas-FasL system has been proposed in cancer cell lines (26) and malignancies in vivo (27, 28). These findings suggest a counterattack against Fas-bearing lymphocytes by FasL-expressing tumor cells in vivo. However, apoptotic signals are also induced by other molecules including TNF α, TRAIL (29), and DF3/MUC1 (30).
Nakashima et al. (3) demonstrated that RCAS1 could induce apoptosis in cultured human lymphoma cells and normal peripheral lymphocytes, which express a RCAS1 receptor. This apoptotic effect might be mediated through activation of caspase genes, because inhibition of these genes strongly abrogated RCAS1-induced apoptosis in vitro (3). RCAS1 protein is secreted into the culture medium of SiSo cells and detected in vaginal discharges of patients with uterine cervical carcinoma but not in those of normal healthy donors (2). In breast carcinomas, Suzuki et al. (31) reported the inverse association of RCAS1/EBAG9 expression and the degree of infiltration of mononuclear cells and CD3+ T cells. Ohshima et al. (32, 33) reported that RCAS1 might have the role of immune evasion in Hodgkin and Reed-Sternberg cells or in uterine glands and cytotrophoblasts. The numerous TIL, mainly T cells, were detected around the border zone between the normal and malignant region in colorectal cancer tissue specimens (not shown). The expression of RCAS1 in colorectal carcinomas cells was associated with the presence of abundant apoptotic TIL compared with tumors, which lacked the expression of this antigen. Although we could not provide a direct evidence that dying cells were TIL, ISNT-positive TIL were identified as round independent cells that have accumulated in the interstitium and were consistent with findings of lymphocytes in HE serial sections. These findings suggest that tumor-derived RCAS1 may induce killing of TIL through the RCAS1-receptor, causing immune cell depletion in human colorectal carcinomas.
CONCLUSION
In conclusion, we have demonstrated immunohistochemically the expression of RCAS1 in advanced colorectal carcinomas. Staining for RCAS1 was detected in tumor cell surface as well as in the cytoplasm, but rarely in normal mucosal glands. The expression of RCAS1 correlated with invasion and lymph node metastases of colorectal tumors. Furthermore, patients with tumors positive for RCAS1 had a significantly poorer prognosis than those negative for this protein. RCAS1 expression correlated with the frequency of apoptotic TIL, suggesting the possible participation of the RCAS1/RCAS1-receptor system in tumor immune escape similar to the Fas/FasL system. Because RCAS1 protein is also secreted and appears to be tumor cell specific, anti-RCAS1 antibody may be useful for serologic diagnosis of colorectal carcinomas. Moreover, blocking RCAS1-mediated killing of TIL by anti-RCAS1 antibody may improve T-cell immune responses to cancer cells.
References
Sonoda K, Nakashima M, Kaku T, Kamura T, Nakano H, Watanabe T . A novel tumor-associated antigen expressed in human uterine and ovarian carcinomas. Cancer 1996; 77: 1501–1509.
Sonoda K, Kaku T, Kamura T, Nakashima M, Watanabe T, Nakano H . Tumor-associated antigen 22-1-1 expression in the uterine cervical squamous neoplasias. Clin Cancer Res 1998; 4: 1517–1520.
Nakashima M, Sonoda K, Watanabe T . Inhibition of cell growth and induction of apoptotic cell death by the human tumor-associated antigen RCAS1. Nat Med 1999; 5: 938–942.
O'Connell J, Bennett MW, O'Sullivan GC, Collins JK, Shanahan F . The Fas counterattack: cancer as a site of immune privilege. Immunol Today 1999; 20: 46–52.
Nagata S . Fas ligand and immune evasion. Nat Med 1996; 2: 1306–1307.
Chappell DB, Restifo NP . T cell-tumor cell: a fatal interaction? Cancer Immunol Immunother 1998; 47: 65–71.
Walker PR, Saas P, Dietrich PY . Role of Fas ligand (CD95L) in immune escape: the tumor cell strikes back. J Immunol 1997; 158: 4521–4524.
Strand S, Galle PR . Immune evasion by tumours: involvement of the CD95 (APO-1/Fas) system and its clinical implications. Mol Med Today 1998; 4: 63–68.
Kubokawa M, Nakashima M, Yao T, Ito K, Harada N, Nawata H, et al. Aberrant intracellular localization of RCAS1 is associated with tumor progression of gastric cancer. Int J Oncol 2001; 19: 695–700.
Noguchi K, Enjoji M, Nakamuta M, Nakashima M, Nishi H, Choi I, et al. Expression of a tumor-associated antigen RCAS1 in hepatocellular carcinoma. Cancer Lett 2001; 168: 197–202.
Kaku T, Sonoda K, Kamura T, Hirakawa T, Sakai K, Amada S, et al. The prognostic significance of tumor-associated antigen 22-1-1 expression in adenocarcinoma of the uterine cervix. Clin Cancer Res 1999; 5: 1449–1453.
Izumi M, Nakanishi Y, Yoshino I, Nakashima M, Watanabe T, Hara N . Expression of tumor-associated antigen RCAS1 correlates significantly with poor prognosis in nonsmall cell lung carcinoma. Cancer 2001; 92: 446–451.
Oshikiri T, Hida Y, Miyamoto M, Hashida H, Katoh K, Suzuoki M, et al. RCAS1 as a tumour progression marker: an independent negative prognostic factor in gallbladder cancer. Br J Cancer 2001; 85: 1922–1927.
Hiraoka K, Hida Y, Miyamoto M, Oshikiri T, Suzuoki M, Nakakubo Y, et al. High expression of tumor-associated antigen RCAS1 in pancreatic ductal adenocarcinoma is an unfavorable prognostic marker. Int J Cancer 2002; 99: 418–423.
Nakakubo Y, Hida Y, Miyamoto M, Hashida H, Oshikiri T, Kato K, et al. The prognostic significance of RCAS1 expression in squamous cell carcinoma of the oesophagus. Cancer Lett 2002; 177: 101–105.
Koji T . Nonradioactive in situ nick translation: a useful molecular histochemical tool to detect single-stranded DNA breaks. Acta Histochem Cytochem 1996; 29: 71–79.
Hashimoto S, Koji T, Kohara N, Kanematsu T, Nakane PK . Frequency of apoptosis relates inversely to invasiveness and metastatic activity in human colorectal cancer. Virchows Arch 1997; 431: 241–248.
Hashimoto S, Koji T, Niu J, Kanematsu T, Nakane PK . Differential staining of DNA strand breaks in dying cells by non-radioactive in situ nick translation. Arch Histol Cytol 1995; 58: 161–170.
Okada K, Komuta K, Hashimoto S, Matsuzaki S, Kanematsu T, Koji T . Frequency of apoptosis of tumor-infiltrating lymphocytes induced by Fas counterattack in human colorectal carcinoma and its correlation with prognosis. Clin Cancer Res 2000; 6: 3560–3564.
Heppner GH . Cell-to-cell interaction in regulating diversity of neoplasms. Semin Cancer Biol 1991; 2: 97–103.
Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM . Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol 1997; 182: 318–324.
Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998; 58: 3491–3494.
Suda T, Takahashi T, Goldstein P, Nagata S . Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993; 75: 1169–1178.
Suda T, Nagata S . Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med 1994; 179: 873–879.
Shukuwa T, Katayama I, Koji T . Fas-mediated apoptosis of melanoma cells and infiltrating lymphocytes in human malignant melanomas. Mod Pathol 2002; 15: 387–396.
O'Connell J, O'Sullivan GC, Collins JK, Shanahan F . The Fas counterattack. Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med 1996; 184: 1075–1082.
Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French LE, et al. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science 1996; 274: 1363–1366.
Bennett MW, O'Connell J, O'Sullivan GC, Brady C, Roche D, Collins JK, et al. The Fas counterattack in vivo: apoptotic depletion of tumor-infiltrating lymphocytes associated with Fas ligand expression by human esophageal carcinoma. J Immunol 1998; 160: 5669–5675.
Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995; 3: 673–682.
Gimmi CD, Morrison BW, Mainprice BA, Gribben JG, Boussiotis VA, Freeman GJ, et al. Breast cancer-associated antigen, DF3/MUC1, induces apoptosis of activated human T cells. Nat Med 1996; 2: 1367–1370.
Suzuki T, Inoue S, Kawabata W, Akahira J, Moriya T, Tsuchiya F, et al. EBAG9/RCAS1 in human breast carcinoma: a possible factor in endocrine-immune interactions. Br J Cancer 2001; 85: 1731–1737.
Ohshima K, Muta K, Nakashima M, Haraoka S, Tutiya T, Suzumiya J, et al. Expression of human tumor-associated antigen RCAS1 in Reed-Sternberg cells in association with Epstein-Barr virus infection: a potential mechanism of immune evasion. Int J Cancer 2001; 93: 91–96.
Ohshima K, Nakashima M, Sonoda K, Kikuchi M, Watanabe T . Expression of RCAS1 and FasL in human trophoblasts and uterine glands during pregnancy: the possible role in immune privilege. Clin Exp Immunol 2001; 123: 481–486.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okada, K., Nakashima, M., Komuta, K. et al. Expression of Tumor-Associated Membrane Antigen, RCAS1, in Human Colorectal Carcinomas and Possible Role in Apoptosis of Tumor-Infiltrating Lymphocytes. Mod Pathol 16, 679–685 (2003). https://doi.org/10.1097/01.MP.0000074732.17945.6C
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.MP.0000074732.17945.6C
Keywords
This article is cited by
-
Apoptotic function of tumor-associated antigen RCAS1 in oral squamous cell carcinoma
Journal of Translational Medicine (2014)
-
The role of RCAS1 as a biomarker in diagnosing CRC and monitoring tumor recurrence and metastasis
Tumor Biology (2014)
-
Cancer therapy using tumor-associated antigens to reduce side effects
Clinical and Experimental Medicine (2009)
-
Clinical Significance of Tumor-associated Antigen RCAS1 Expression in Human Pancreatic Ductal Adenocarcinoma
Digestive Diseases and Sciences (2008)
-
Prognostic Significance of Receptor-Binding Cancer Antigen Expressed on SiSo Cells (RCAS1) Expression in Relation to Cadherin Expression in Patients with Colorectal Carcinoma
Diseases of the Colon & Rectum (2007)